17.3: Properties Of Alcohols And Phenols
Di: Jacob
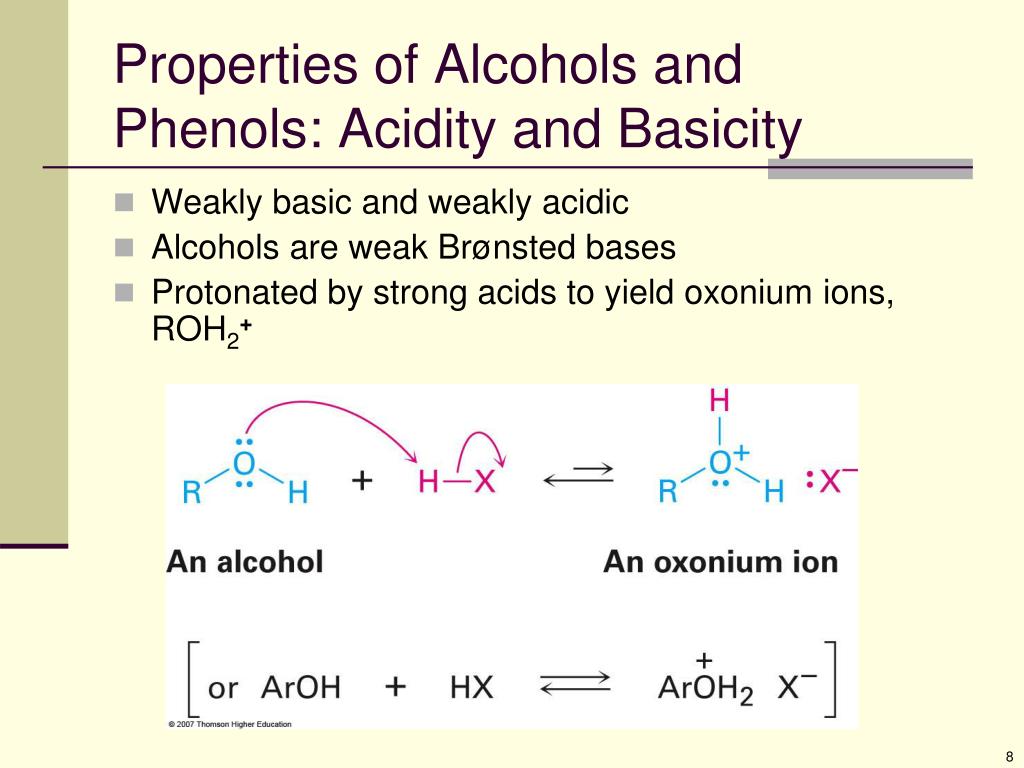
2: Properties of alcohols and phenols: Hydrogen bonding: The structure around the oxygen atom of an alcohol or phenol is similar to that in water and is sp 3 hybridized5 Alcohols from Carbonyl Compounds: Grignard Reaction; 17. list a given series of alcohols or phenols in order of increasing or decreasing acidity.3: Properties of Alcohols and Phenols – Chemistry LibreTexts2: Physical Properties of Alcohols and Phenols.2 Properties of Alcohols and Phenols., H-bonding must be destroyed to boil.8 Protection of Alcohols; 17. Phenols are more acidic than alcohols .17 Properties of Alcohols and Phenols. This table shows that alcohols (in blue) have higher boiling points than haloalkanes and alkanes with the . The R–O–H bond angle has an . Recall that S N 1 reactions are promoted in polar, protic solvents. Simple alcohols are named in the IUPAC system as derivatives of the parent alkane, using the suffix – ol. Oxymercuration – demercurationis a special electrophilic addition (Section 8.3: Properties of alcohols and phenols: acidity and basicity: Like water alcohols are weak Brønsted bases Electronic factors that . It is syn-stereospecific and regioselective.It is anti-stereospecific and regioselective. Spin–spin splitting, however, is not usually observed between the O–H proton of an alcohol and the .3: Properties of alcohols and phenols: acidity and basicity: Like water alcohols are weak Brønsted bases Electronic factors that influence acidity:.2 Properties of Alcohols and Phenols Properties of Alcohols and Phenols: Acidity and Basicity Alcohols and Phenols are Weak Brønsted Acids Acidity Measurements pKa Values for Typical OH Compounds Relative Acidities of Alcohols Inductive Effects .comEmpfohlen auf der Grundlage der beliebten • Feedback
Chapter 17: Alcohols and Phenols
A complete summary, study notes and related key terms to know for Organic Chemistry Unit 17 – Alcohols and Phenols.An example of this is seen when comparing 1-propanol (MW = 60.Tertiary alcohols.

Chapter 17: Alcohols and Phenols
Alcohols are prepared by S N 2 reaction.Naming Alcohols and Phenols. Alcohols, enols and phenols all have significantly higher boiling points than alkanes, due to hydrogen bonding. Alcohols are organic compounds in which a hydroxy group is attached to a saturated carbon.Empfohlen auf der Grundlage der beliebten • Feedback[PDF] Chapter 17: Alcohols and Phenols.
Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of organic groups bonded to the hydroxyl-bearing carbon. Alcohols from Carbonyl Compounds: Grignard Reaction. Phenols don’t undergo oxidation in the same way as alcohols because they don’t have a hydrogen atom on the hydroxyl-bearing carbon.

The R–O–H bond angle has an approximately tetrahedral value (108. Primary and secondary alcohols are much more resistant to .
Physical Properties and Uses of Alcohols and Phenols (Video)
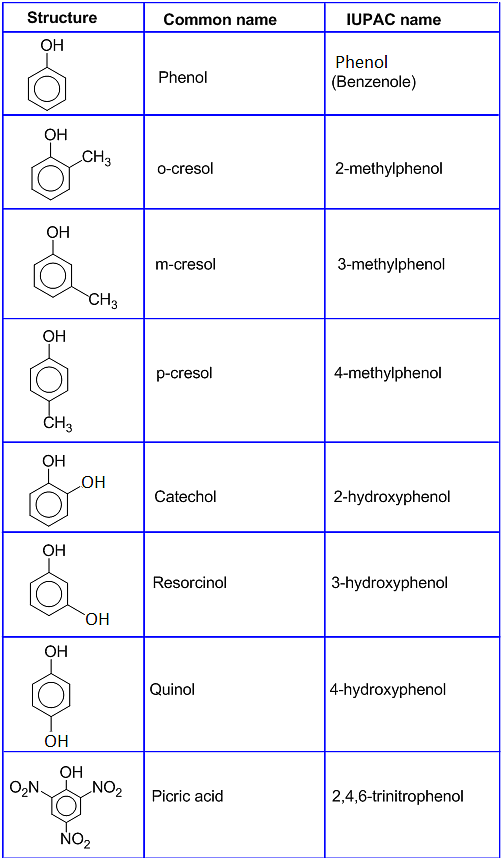
Select the longest carbon chain containing the . RULE 1 Select the longest carbon chain containing the hydroxyl group, and derive the parent name by replacing the -e ending of the corresponding alkane with -ol. The S N 2 reaction of an alcohol with an alkyl halide proceeds with two inversions of configuration—one to make the halide from the alcohol and one to substitute the halide—and yields a product with the same stereochemistry as the starting alcohol. Alcohols contain an OH group bonded to.One of the most important reasons for using tosylates in S N 2 reactions is stereochemical.2: Naming Alcohols and Phenols.Alcohols also show characteristic absorptions in the 1 H NMR spectrum.Lecture Outline. We also introduce the concept of protecting a sensitive functional group during an organic .2 • Structure and Properties of Carboxylic Acids Carboxylic acids are similar in some respects to both ketones and alcohols.2 Properties of Alcohols and Phenols: Hydrogen Bonding alcohols, phenols, ethers are H 2 O derivatives Hydrogen bonding: numbering: begin at the end nearer the OH
Chapter 17
In a tertiary (3°) alcohol, the carbon atom holding the -OH group is attached directly to three alkyl groups, which may be any combination of same or different. The reaction proceeds in an Anti-Markovnikov manner, where the hydrogen (from BH 3 or BHR 2) attaches to the more substituted carbon and the boron attaches to the least substituted carbon in the alkene double bond.1) which have boiling points of 94. The physical properties of the alcohols and phenols are influenced by hydrogen bonding due to the oxygen–hydrogen .4 The central position of alcohols in organic chemistry.Alcohols and phenols have nearly the same geometry around the oxygen atom as water.discuss the factors that are believed to determine the acidity of alcohols and phenols.As a result, phenols are highly reactive substrates for electrophilic halogenation, nitration, sulfonation, and Friedel–Crafts reactions.1), Chloroethane (MW = 64.Hydroboration-Oxidation is a two step pathway used to produce alcohols (Section 8. Small alcohols .2 • Properties of Alcohols and Phenols Alcohols and phenols have nearly the same geometry around the oxygen atom as water. Reactions of Alcohols . DEFINITION, STRUCTURE & PHYSICAL PROPERTIES OF ALCOHOLS (17. Hydrogens on the oxygen-bearing carbon atom are deshielded by the electron-withdrawing effect of the nearby oxygen, and their absorptions occur in the range 3.3 Physical Properties of Alcohols – Lumen Learningcourses. Instead, oxidation of a phenol yields a 2,5-cyclohexadiene-1,4-dione .2) Alcohols are compounds which contain a hydroxy group bonded . Phenols are a class of alcohols containing a hydroxy group attached to an aromatic ring. Factors that influence acidity: Inductive effect: alcohol a stronger acid by stabilizing .eduLAB Report Experiment 1 Reactions OF Alcohol AND Phenolsstudocu.If one H is replaced by an alkyl group (R-), the compound is an alcohol (R-OH) and if the H of water is replaced by an aromatic group (Ar-), the compound is a phenol (Ar-OH). Boiling points: alcohols > chloroalkanes > alkanes .1 • Naming Alcohols and Phenols Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of organic groups bonded to the hydroxyl .9 • Phenols and Their Uses The outbreak of World War I provided a stimulus for the industrial preparation of large amounts of synthetic phenol, which was needed as a raw .Simple alcohols are named in the IUPAC system as derivatives of the parent alkane, using the suffix -ol.A water molecule then attacks the most substituted carbon to open the mercurium . Substrates that undergo substitution by SN1 reaction can be converted to alcohols using water as the nucleophile (and it can even be the solvent).Alcohols are organic molecules containing the functional hydroxyl or –OH group directly bonded to carbon.Chapter 17: Alcohols and Phenols Based on McMurry’s Organic Chemistry, 7th edition2 Properties of Alcohols and Phenols: Hydrogen Bonding alcohols, phenols, ethers are H2O derivatives Hydrogen bonding: numbering: begin at the end nearer the OH O R H H O H R O R δ− δ− δ − δ+ δ+ δ+ H-bonding: 5-10 kcal/mol – alcohols, phenols: high b.Alcohols occupy a central position in organic chemistry.The discussion begins with an outline of the nomenclature of alcohols and phenols.Alcohols from Alkenes. c) CH 3 CH 2 CH 2 OH.Chapter 17: Alcohols and Phenols Based on McMurry’s Organic Chemistry, 6th edition ©2003 Ronald Kluger Department of Chemistry University of Toronto Preparation of Alcohols: A Review.In this chapter, we concentrate on the oxidation of alcohols to carbonyl compounds.4 Alcohols from Carbonyl Compounds: Reduction; 17.1 Naming Alcohols and Phenols; 17.2 Properties of Alcohols and Phenols; 17.5° in methanol, for . Small alcohols are soluble in water. Alkyl halides can be converted to alcohols by using S N 2 reactions with OH-as a nucleophile.9 Phenols and Their Uses; .chrome_reader_mode Enter Reader Mode . Phenols have an OH group directly attached to a benzene ring. [PDF] 120 Chapter 24: Phenols. The -e is deleted to prevent the occurrence of two adjacent vowels: propanol rather than .2 Properties of Alcohols and Phenols – Chemistry .5: Substituent Effects on the Acidity of .11 • Spectroscopy of Alcohols and Phenols Infrared Spectroscopy.Tertiary alcohols react with either HCl or HBr at 0 °C by an S N 1 mechanism through a carbocation intermediate.3 Preparation of Alcohols: A Review; 17. They can be prepared from many other kinds of compounds (alkenes, alkyl halides, ketones, esters, and aldehydes, among others), and they can be transformed into an equally wide assortment of compounds ( Figure 17. A Representation of Hydrogen Bonding in Alcohols.1 Naming Alcohols and Phenols IUPAC Rules for Naming Alcohols Naming Phenols 17. While alcohols are colorless, phenol is a white crystalline compound with a characteristic hospital smell odor. Alcohols have a strong C–O stretching absorption near 1050 cm –1 and a characteristic O–H stretching .2: Properties of alcohols and phenols: Hydrogen bonding: The structure around the oxygen atom of an alcohol or phenol is similar to that in water and is sp3 hybridized Alcohols and phenols have much higher boiling points than similar alkanes and alkyl halides H 2O CH 3CH 2CH 2CH 3! O CH 3CH 2CH 2CH 2Cl CH 3CH 2CH 2OH MW=18 . Indicate if the following molecules are 1 o, 2 o, or 3 o alcohols: 1) a) b) (CH 3) 3 COH. Alcohols from Carbonyl Compounds: Reduction.

As with other organic compounds, alcohols and phenols are .
Organic Chemistry Chapter 17: Alcohols & Phenols
3 o C, and -1 o C respectively. Properties of Alcohols and Phenols.Chapter 17: Alcohols and Phenols – Vanderbilt Universityvanderbilt. Like ketones, the carboxyl carbon is sp 2 -hybridized, and carboxylic acid groups are therefore planar with C–C═O C–C═O and O═C–O O═C–O bond angles of approximately 120° ( Table 20.

6 Reactions of Alcohols; 17.

This reaction involves mercury undergoing electrophilic addition to the alkene double bond forming a Mercurinium Ion Bridge. We review the physical properties of these compounds, and discuss methods used to obtain .

7 Oxidation of Alcohols; 17. Examples: Exercise 17. Alcohols are amphoteric, they act as an acid when reacting with bases and as a base when reacting with acids.5), and butane (MW = 58.
- Cvn-68 Uss Nimitz 1975 Modellbausatz
- Passstrasse Über Den Gotthard Wieder Befahrbar
- Sonic Health Care Gmbh – Our Divisions
- Kenwood Reparatur In Der Nähe | Professionelle Fachwerkstätten für Hifi-Geräte Reparatur
- Samsung Galaxy A34 5G Passwort / Muster Vergessen
- Zuzahlung Bei Pflege , Durchschnittliche PFLEGEKOSTEN im PFLEGEHEIM 2024
- Aufkochen Synonym-Lexikothek • Ein Anderes Wort Für Aufkochen
- Les Enfants Et Le Divorce : Stratégies Pour Minimiser L’Impact
- Medizinstudium In Bulgarien I Bewerbung
- Brændte Mandler Med Sukrin : Brændte Mandler
- Slytherins Find Out Harry Is Abused
- Den Klang Der Menschlichen Stimme Sichtbar Machen
- Té Negro Como Tinte Casero Para Cubrir Las Canas
- If Guru Was Such A Highly Respected Rapper, Why Did He Never