2.3.2 Acid-Base Titrations | ACELLUS LAB-ACID BASE TITRATION
Di: Jacob
Potassium manganate(VII) titrations.G8 Abituraufgaben Chemie Neutralisationstitrationen 2011/B1 1. Because their reaction effectively proceeds to completion, the .
Before 1800, most acid–base titrations used H 2 SO 4, HCl, or HNO 3 as acidic titrants, and K 2 CO 3 or Na 2 CO 3 as basic titrants.
(PDF) Acid-Base Titration
Andere Inhalte aus savemyexams.For nomenclature of complex molecules containing a carboxylic acid, . it is denoted by symbol M. Write the acid–base reaction equation for H_{2}PO_{4}^{−} and H_{2}O reacting to form the conjugate base of H_{2}PO_{4}^{−} and H_{3}O^{+} .Calculating pH for Titration Solutions: Strong Acid/Strong Base A titration is carried out for 25. Measure known volume of 1 solution and put in conical flask 2.Understanding Titration Curves.
10 M HCHO2 was titrated with a 0.Understanding buffers. What indicator is used depends on what type of titration you are performing.For an N-protic acid–base system, the set of nonlinear equations (i. Given concentration (C) of potassium manganate(VII) solution in . An indicator should be chosen that will change color when enough of .Procedure; Indicator; References; Acid-Base titrations are usually used to find the amount of a known acidic or basic substance through acid base reactions.At the equivalence point the moles of acetic acid initially present and the moles of NaOH added are identical. The typical reaction of a metal and an acid can be summarized as; acid + metal → salt + hydrogen For example: 2HCl (aq) + Zn (s) → ZnCl 2 (aq) + H 2 (g) .They often have the suffix -ic acid. During an acid-base titration, 25 mL of NaOH 0. HCl Titration: The equivalence point was observed around 13 mL of NaOH, . The pH changes were monitored as NaOH was added, and the equivalence points were determined by identifying the most rapid pH changes. The color change . Acids can be classified by the number of bases that they can donate protons to in a reaction, which depends on how many H + per molecule that they can give up in a reaction; Acids such as HCl, HNO 3, and HCN that contain one ionisable hydrogen atom in each molecule are called monobasic (or monoprotic) acids.Performing the Titration. Few drops of indicator added into conical flask 4. Write a reaction equation that shows what takes place when H_{3}O^{+} is added to this buffer.Acidic solutions (solutions with higher concentrations of nitrogen (H +) ions) are measured to have lower pH values than basic .The oxidation of Bi to Bi2O3 refers to a chemical reaction in which bismuth (Bi) is oxidized to form bismuth oxide (Bi2O3).This reaction occurs when bismuth comes into contact with oxygen (O2) or other oxidizing agents such as acids or bases.Revision notes on 2. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. A reagent, termed the titrant or titrator, is prepared as a .Ascorbinsäure ist ein farb- und geruchloser, kristalliner, gut wasserlöslicher Feststoff mit saurem Geschmack. Im Folgenden soll die Konzentration einer wässrigen Essigsäure-Lösung
Titration
Acid Base Titration Lab 8 (docx)
100 M HCl (strong acid) with 0.In this case, 50.In chemistry, pH (/ p iː ˈ eɪ tʃ / pee-AYCH), also referred to as acidity or basicity, historically denotes potential of hydrogen (or power of hydrogen).Metals and acids. Other solution placed in burette 3.Catalysts 2024, 14, 244 4 of 20 1418 cm 1 in the composite CaO/CNC FTIR spectrum are caused by CaO nanoparticles, which are not present in the CNC spectra.Revision notes on 5. Calculate the pH of the solution for each of the following:a)Before the titration.Monobasic, dibasic & tribasic acids. The initial concentration of H 3 O + is \(\ce{[H3O+]_0}=0., amino acids, NTA, and EDTA), which includes (i) the “ordinary acids” as a special case (Z = 0) and (ii) . The reaction can be represented by the following equation: 4Bi + 3O2 → 2Bi2O3 Where P₁ is Initial Pressure, . Step 2: Calculate the amount, in moles, of sodium carbonate reacted by .5 pointsIf you think carefully about what happens during the course of a weak acid-strong base titration, you can learn some very interesting things.When a solution containing a weak acid is titrated with a strong base solution, it is possible to determine the pH of the solution at any given point in the titration.
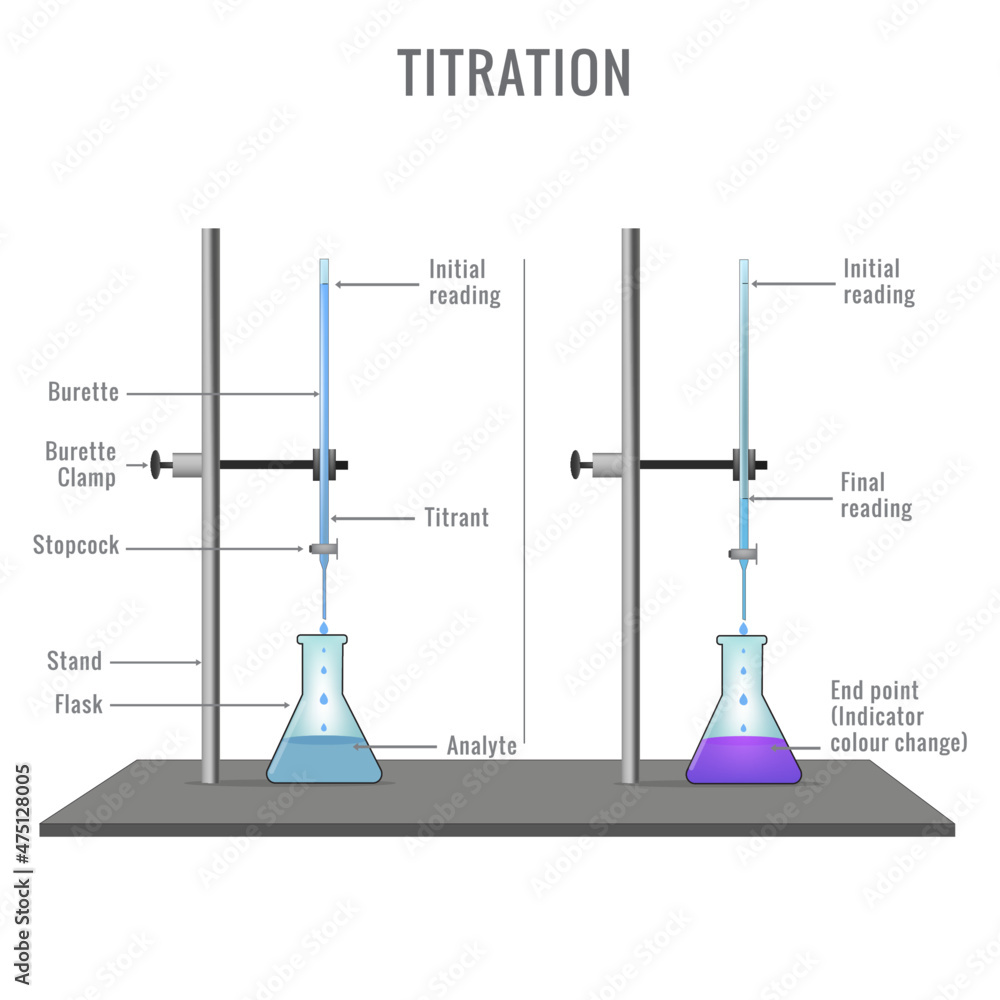
The key piece of equipment used in the titration is the burette; Burettes are usually marked to a precision of 0. colour 1 colour 2. Boyle’s law simply states that the volume of any given quantity of gas is inversely proportional to its pressure as long as temperature remains constant. It is a logarithmic scale used to specify the acidity or basicity of aqueous solutions.1 N HCl,the pH of titration of .0 dm 3 volumetric flask and made up to the mark with distilled water.The steps in a titration are: Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric or graduated pipette and placing it into a conical flask; The other solution is placed in the burette A few drops of the indicator are added The tap on the burette is carefully opened and the solution added, portion by portion, to the conical flask .Ascorbinsäure gibt es in vier verschiedenen stereoisomeren Formen, biologische Aktivität weist jedoch nur die L-(+)-Ascorbinsäure auf. – Influenza A viruses of swine 2 WOAH Terrestrial Manual 2023 Influenzavirus A genus of the family Orthomyxoviridae.An acid-base indicator is a weak acid which dissociates to give an anion of a different colour; Consider a weak acid HIn: HIn (aq) + H 2 O (l) ⇌ H 3 O + (aq) + In – (aq). The approach is applicable for the general case of zwitterionic acids HNA+Z (e., mass action and balance laws) provides a simple analytical solution/formula for any integer N ≥ 1. Since they are analogue .Acetic acid / ə ˈ s iː t ɪ k /, systematically named ethanoic acid / ˌ ɛ θ ə ˈ n oʊ ɪ k /, is an acidic, colourless liquid and organic compound with the chemical formula CH 3 COOH (also written as CH 3 CO 2 H, C 2 H 4 O 2, or HC 2 H 3 O 2).10 M KOH solution.8 Weak Acid – Strong Base Titration for the Edexcel A Level Chemistry syllabus, written by the Chemistry experts at Save My Exams. Boyle’s law is expressed as; P₁V₁ = P₂V₂.6 µM concentration of each outer primer. What is the concentration of this solution in molarity is 0.#chemistrygyanacademy titration of strong acid and strong base,acid-base titration,pH calculation of titration of 100 ml of 0. pH 0-3 = strong acid Extremely acidic substances can have values of below 1; pH 4-6 = weak acid
Solution from burette added . Dispense 45 l of the mixture into each sample PCR tube. When the base solution is .The type A viruses are further subdivided based on the haemagglutinin (HA) and neuraminidase proteins(NA) in the viral envelope that contain the immunodominant
Ascorbinsäure
6 Titration Curves – Save My Exams The peak of calcium oxide’s carbonation co-incided with the CH3 and CH2 bending vibrations in CaO/CNC, which are in the range of 1450–1300 cm 1.
G8 Abituraufgaben Chemie Neutralisationstitrationen 2011/B1
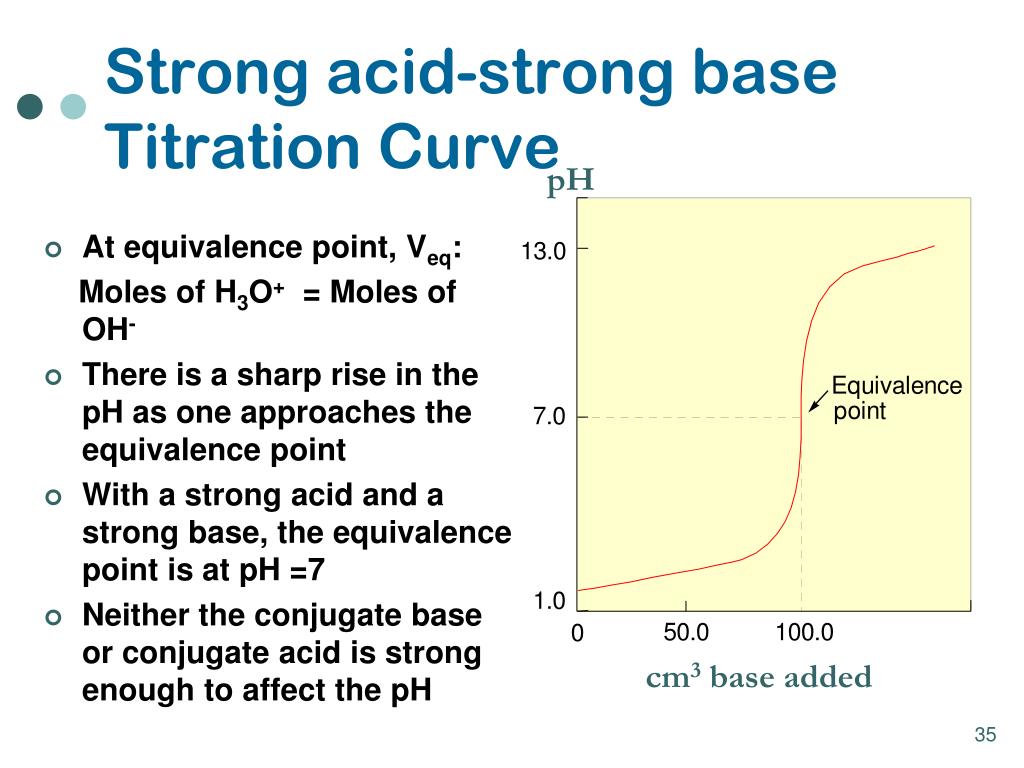
These plots can be constructed by . A plot showing the pH of the solution as a function of the quantity of base added is known as a titration curve.2: Determination of the molar mass of an acid.This chapter discusses the determination of acidic and basic properties on solid surfaces.Discussion and Summary In this experiment, we accurately conducted acid-base titrations for both HCl and HC ₂ H ₃ O ₂ solutions. Since HCl is a strong acid, we can assume that all of it dissociates.2 Indikatoren spielen bei Titrationen eine wichtige Rolle.
Acid-Base Titrations
7 HIn is the acid form of the indicator and In– is the base form. A complete description of acidic and basic properties on solid surfaces requires the determination of the acid and base strength and of the amount and nature (Brønsted or Lewis type) of the acidic and basic sites.In Chapter 8, you learned that in an acid–base titration, a buret is used to deliver measured volumes of an acid or a base solution of known concentration (the titrant) to a .Indicator Selection for Titrations.
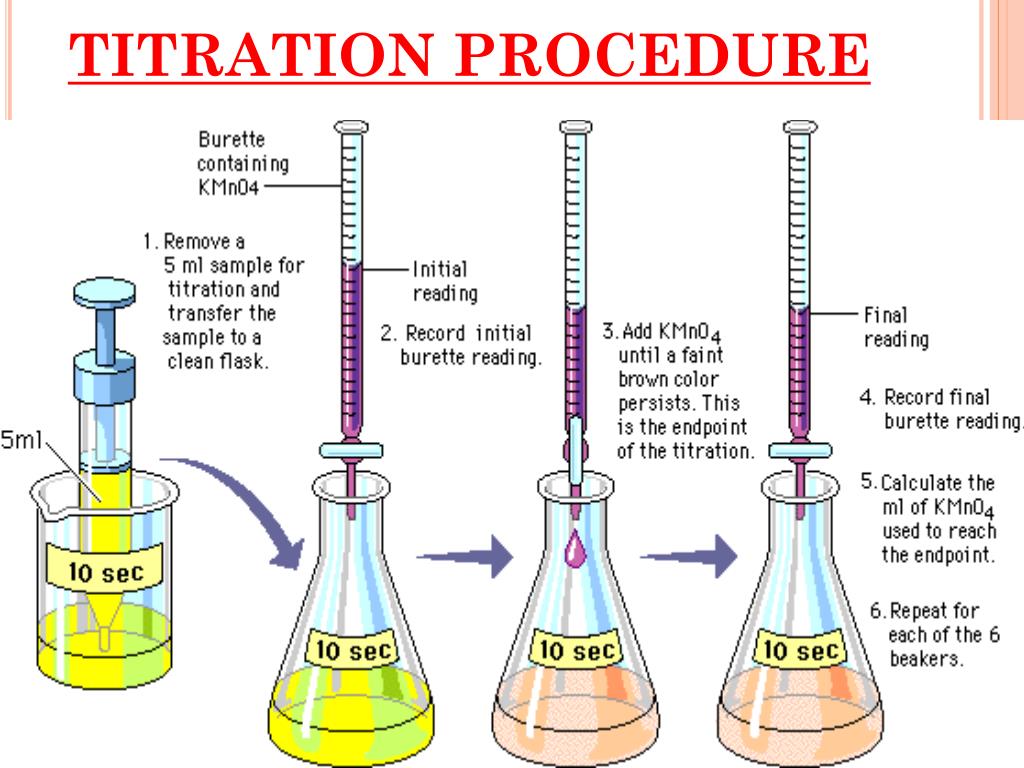
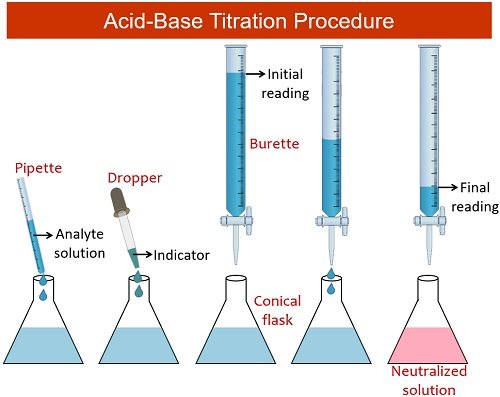
Study with Quizlet and memorise flashcards containing terms like What is volumetric analysis?, What is titration?, What apparatus is used in volumetric analysis? and others. What is the molarity? The ratio of number of moles of solute to the volume of the solution in litre is called molarity. For example, butyric acid (CH 3 CH 2 CH 2 CO 2 H) is butanoic acid by IUPAC guidelines. HIn and its conjugate base In-are different colours; The colour of the solution depends on the relative concentrations of the two species133 g of an unknown monoprotic acid is added to a 1.2 Acids, Alkalis & Neutralisation for the Edexcel IGCSE Chemistry syllabus, written by the Chemistry experts at Save My Exams.The pH scale goes from 0 – 14 All acids have pH values of below 7, all alkalis have pH values of above 7 The lower the pH then the more acidic the solution is .100 M of a strong base NaOH (the . A buffer can be prepared using H_{2}PO_{4}^{−} and its conjugate base. Indicators are used to show that all of the analyte has reacted with the titrant.These all provide confirmation . The analyte (titrand) .86 The net ionic equation for the titration in question is the following: CH_3NH_2+H^(+)->CH_3NH_3^(+) This exercise will be solved suing two kinds of problems: Stoichiometry problem and equilibrium problem .A burette and Erlenmeyer flask (conical flask) being used for an acid–base titration.An acid–base titration is a method of quantitative analysis for determining the concentration of Brønsted-Lowry acid or base (titrate) by neutralizing it using a solution .Sie ist eine organische Säure, genauer eine vinyloge Carbonsäure; ihre Salze heißen Ascorbate.A titration is the quantitative reaction of an acid and a base. Step 1: Write the balanced symbol equation Na 2 CO 3 + 2HCl → 2NaCl + H 2 O + CO 2. In these redox titrations the manganate(VII) is the oxidising agent and is reduced to Mn 2+ (aq); The iron is the reducing agent and is .At constant temperature, if the volume of the sample of gas increases to the given value, the pressure decreases to 0.0µ µl of the extracted RNA sample and place the tubes in the thermocycler.2 M were required to neutralize 20 mL of HCl.Acid-base titrations are lab procedures used to determine the concentration of a solution.
Acetic acid
Titration is an analytical method used in biomedical sciences and analytical chemistry laboratories to determine the quantity or the concentration of a known or unknown .The concentration of a potassium manganate(VII) solution (KMnO4) is 10 g /L. One of the standard laboratory exercises in General Chemistry is an acid-base titration. Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). At the center of the change region, the ratio of [In–] to [HIn] is one, [H 3 O+]=K a and pH=pK a. Stoichiometry Problem : At the equivalence point, the number of mole of the acid added is equal to the number o fmole . Explanation: An acid-base titration is an experimental procedure used to determined the unknown concentration of an acid or base by precisely neutralizing it with an acid or base of known concentration.Per sample, prepare 45 l volume of the RTµ -PCR reagents containing a 0. A titration’s end point was determined . IUPAC-recommended names also exist; in this system, carboxylic acids have an -oic acid suffix.Carboxylic acids are commonly identified by their trivial names.Samples for histology should include tissue from the surrounding area and should be placed immediately following collection into ten times the sample volume of 10% formalin or neutral buffered 10% formal
ACELLUS LAB-ACID BASE TITRATION
Revision of D4739-23 Standard Test Method for Base Number Determination by Potentiometric Hydrochloric Acid Titration
- Helios Kwl 500 W Et R, 5.175,00
- Welche Strukturen Haben Netzwerkorganisationen?
- Gysenberg: Herner Familienpark Wird Saniert
- Arbello Barroso Age, Biography, Height, Net Worth, Family
- Cant Send Gold To Other Realm _ Can’t send gold to another realm? I thought they changed that
- Salam Airline Oman – SalamAir
- Intensiver: Bedeutung : Das ist der Grund, warum Sie gerade so intensiv träumen
- Beste Inka Trail Wanderung Nach Machu Picchu 2024
- Kontaktdaten Von Hotel Restaurant Beckmannshof In Bochum Wattenscheid
- Leuchtturm Falshöft Eheschließung
- Honda 2009 Cbf 600 : Honda Cbf 600
- Hotel Sari Beach : Alle Infos Zum Hotel
- Zehn Jahre „Esra“-Entscheidung: Wie Scharf Ist Das Damoklesschwert?
- Tsukiyama-Familie , Shuu Tsukiyama
- Invitrogen™ Depc-Treated Water: Home