5.6: Balancing Redox Reactions
Di: Jacob
Oxidation-Reduction Reactions Figure 1 Comparing oxidizing and reducing agents. Conversely, the C causes the Fe 2 O 3 to lose oxygen . Identify the substance oxidized, substance reduced, reducing agent and reducing agent.It is the ultimate source of all food on Earth, and it is a redox reaction. No redox occurs, and the product, lead iodide, precipitates from the solution as a bright yellow solid . Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions.Balancing Redox Reactions – The Half-Reaction Method. You should be able to predict the charge of an aluminum ion using the periodic table. O 2 − + F 2 → O 2 + F −.4 – Balancing Redox Reactions in Basic Solution .1 Redox Reactions and Oxidation Numbers.For balancing Redox reactions, it is necessary to first balance the main atoms (through adjusting stoichiometric coefficients), then the charges (through electron transfer and as .3 Galvanic Cells. Appendix C: Units and Conversion . Coupled equilibria (solubility, complexation, acid-base, and other reactions) change the value of E°, effectively by changing the concentrations of free metal ions.Oxidation-Reduction Reactions, or redox reactions, are reactions in which one reactant is oxidized and one reactant is reduced simultaneously. This module demonstrates how to .Here are some examples for practice.In MgCl 2, magnesium has an oxidation number of +2, while chlorine has an oxidation number of −1 by rule 2. To balance a redox reaction, first take an equation and separate .Demonstrations › Chemical Reactions II › 5.5 Other Functional .6: Recognizing Redox Reactions
Balancing redox equations (article)
Single-displacement reactions are reactions of metals with either acids or another metal salt that result in dissolution of the . Oxidation numbers are used to keep track of electrons in atoms.Balancing Redox Reactions via the Half-Reaction Method. Because a balanced chemical equation is the most important prerequisite for solving any stoichiometry problem, we need a method for balancing oxidation–reduction reactions in aqueous solution .2: Balancing Redox Reactions In studying redox chemistry, it is important to begin by learning to balance electrochemical reactions. We can use the Nernst equation to calculate the value of E° from the equilibrium constant for the coupled reaction. However, when we compare the overall charges on each side of .5 Batteries and Fuel Cells. Simple redox reactions (for example, H 2 + I 2 → 2 HI) can be balanced by .Twa worksheet 2. The ion-electron method allows one to balance redox reactions regardless of . Also, check CBSE Class 11 Chemistry revision notes for other chapters: CBSE Class 11 Chemistry Chapter-wise . Give some biochemical examples of oxidation and reduction reactions. Balance oxygen atoms by adding H .
Revision Notes Class 11 Chemistry Ch 8
There exists multiple techniques to balancing redox reactions, one of which is presented below (called the half-reaction method).Check Your Learning 1.We can balance oxidation–reduction reactions in solution using the oxidation state method (Table \(\PageIndex{1}\)), in which the overall reaction is separated into an oxidation equation and a reduction equation. The source of oxygen is Fe 2 O 3.pdf – TRUE WAY ASL Workbook Unit 5. Redox reactions are common in organic and biological chemistry, including the combustion of organic chemicals, respiration, and photosynthesis. Simple redox reactions can be balanced by inspection, but for more complex reactions it is helpful to have a foolproof, systematic method. Chapter 18 – Review. Appendix B: Essential Mathematics.Balancing Redox Reactions.

7 Electrolysis. Balance all elements except oxygen and hydrogen.Write and balance the redox reaction that has calcium ions and potassium metal as reactants and calcium metal and potassium ions as products.Balance the following in an acidic solution. Zinc is a strong reducing agent and is capable of reducing both Cr 2 O 72- to Cr 3+ and Cr 3+ to .

Balancing redox reactions by the ion-electron methodperiodni. Oxidation and reduction always occur together, even though they can be written as separate chemical equations. 2 O 2− + 2 F 2 → O 2 + 4 F −.Balance the redox reaction by oxidation number method N2H4+ClO3- gives NO+Cl- in (basic medium) View Solution. Identify the substance . The balanced reduction half reaction is as follows: \[\ce{2H^{+} + 2e^{−} → H2} \nonumber \]2 Branched Hydrocarbons. Oxidation: 2 (K → K + + e −) Combined: Ca 2 + + 2K → Ca + 2K + The . Concept Review Exercise. An oxidation-reduction reaction, or redox reaction, is a reaction that involves the full or partial transfer of electrons from one reactant to another.Identify the oxidant and reductant of a redox reaction; Balance chemical equations for redox reactions using the half-reaction method3 – Balancing Redox Reactions in Acidic Solution In acidic solution, hydrogen peroxide reacts with Fe 2+ to produce Fe 3+ and H 2 O. A redox titration involves an oxidising agent being titrated against a reducing agent; Electrons are transferred from one species to another; In acid-base titrations indicators are used to show the endpoint of a reaction; however redox titrations using transition metal ions naturally change colour when changing oxidation . Another method for balancing redox reactions uses half-reactions. Chapter 18 – Summary. SO2 − 3 (aq) + MnO − 4 (aq) → SO2 − 4 (aq) + Mn2 + (aq) Solution. We start by using symbols of the elements and ions to represent the reaction: Ag + + Al → Ag + Al 3 + The equation looks balanced as it is written.4 Other Oxygen-Containing Functional Groups. Many redox reactions occur in aqueous solution—in water. We start by using symbols of the elements and ions to represent the .Neutralization is the reaction of an acid and a base, which forms water and a salt.To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: an oxidation—in which the atoms of one element lose .1 Hydrocarbons . Appendix A: The Periodic Table. There are rules . Reduction is the gain of electrons. Write a balanced equation for this reaction.This method assumes that the redox reaction is taking place in . Net ionic equations for neutralization reactions may include solid acids, solid bases, solid salts, and water. As a general rule, if you balance the .orgEmpfohlen auf der Grundlage der beliebten • FeedbackIn H 2 O, the H atoms each have an oxidation number of +1, while the O atom has an oxidation number of −2, even though hydrogen and oxygen . H 2 O 2 (aq) + 2 H + (aq) + 2 Fe 2 + → 2 H 2 O (l) + 2 Fe 3+. In the above equation, the overall charge is zero, or neutral, on both sides of the equation.When oxidation–reduction reactions occur in aqueous solution, however, the equations are more complex and can be more difficult to balance by inspection. Subjects: Oxidation/Reduction, Net ionic equations.

For the reaction between lead(II) nitrate and potassium iodide, the products are predicted to be lead(II) iodide and potassium nitrate.Balancing Redox Equations: Half-Reaction Method. Reduction: Ca 2 + + 2e − → Ca.5: Redox Reactions Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions. Redox reactions that take place in aqueous media often involve water, hydronium ions, and hydroxide ions as .
Balancing of Redox Reactions. In other words, Fe 2 O 3 is causing the C to be oxidized.The voltage of a voltaic cell is determined by the difference in the tendencies of the individual half cells and is characteristic of a given redox reaction when concentrations . Download CBSE Class 11 Chemistry Revision Notes 2024-25 PDF.Redox reactions are also commonly described in terms of oxidizing and reducing agents.3 Alkyl Halides and Alcohols.comWorked example: Balancing a simple redox equation – Khan . Description: When a ball of aluminum foil is placed in a copper solution with chloride . Reduction is the loss of oxygen, eg: Cu+ H 2 O → 2CuO + H 2 (O has been reduced) Reduction is also the gain of a hydrogen, eg: 2NH 3 + 3Br 2 → N 2 + 6HBr (Br has been reduced) Reduction is also the gain of electrons, eg: Cu 2+ + Mg → Mg 2+ + Cu (Cu has been reduced) Reduction causes a decrease in oxidation number, eg: Cu 2 . Redox Reactions And Electrode Processes.Redox Reactions with Coupled Equilibria.3 Applications of Redox Reactions: Voltaic Cells.In studying redox chemistry, it is important to begin by learning to balance electrochemical reactions. Balancing by charge means making sure that the overall charge is the same on both sides of the equation. from Wikipedia. Redox reactions require that we keep track of the electrons assigned to each atom in a chemical reaction. Redox reactions involve additional considerations when balancing when compared to balancing regular chemical equations. Oxidation is the loss of electrons. This method assumes that the redox reaction . Recall that a half-reaction is either the oxidation or .
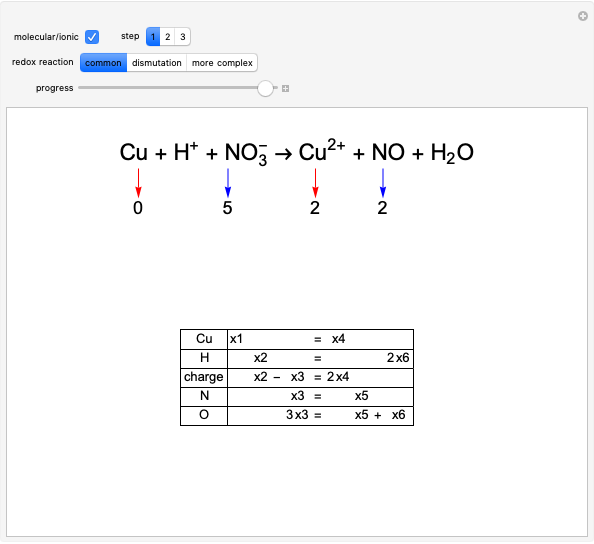
We will concern ourselves with the balancing of these equations at another time. The redox reactions discussed so far tended to be rather simple, and conservation of mass (atom counting by . Balance the following redox equations by oxidation number method occur in acidic medium: H 2 O 2 + C r 2 O 2 − 7 (a q) → O 2 (g) + C r 3 + (a q) View Solution .There exists multiple techniques to balancing redox reactions, one of which is presented below (called the half-reaction method). Source: Jung-Lynn Jonathan Yang.Remember, however, that one of the products must precipitate, otherwise no chemical reaction has occurred.4 Electrolysis. Subjects: Oxidation/Reduction, Net ionic equations Description: When a ball of aluminum foil is placed in a copper solution with chloride ions, the copper ions are reduced to copper .2 Balancing Redox Reactions.4 Electrode and Cell Potentials. Organic Chemistry.With half reactions, there is one more item to balance: the overall charge on each side of the reaction. Because of this, in many cases H 2 O or a fragment of an H 2 O molecule (H + or OH −, in particular) can participate in the redox reaction.Balance this redox reaction by using the half reaction method.Oxidation of Fe 2+ to Fe 3+.

Alternatively, we can measure . If you check each side of this reaction, you will note that both sides have a zero net charge.6 Reduction of Cu 2+ by Aluminum. Reduction is the full or partial gain of electrons or the . Consequently, Fe 2 O 3 is referred to as the oxidizing agent. Limitations of Concept of Oxidation Number.pdf Asl unit true way worksheet comprehension quiz part workbook pdf activity Asl unit 5. Hydrogen is reduced in the reaction. Unit 12 Organic Chemistry. Write the two half-reactions representing the redox process. In H 2, both H atoms have an oxidation number of 0 by rule 1.A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Oxidation is the full or partial loss of electrons or the gain of oxygen.Balancing by mass means ensuring that there are equal masses of each element on the product and reactant sides. (A silver ion will have a plus one charge. In the reaction below, C is being oxidized by gaining oxygen. Chemical Reactions II: Oxidation/Reduction.
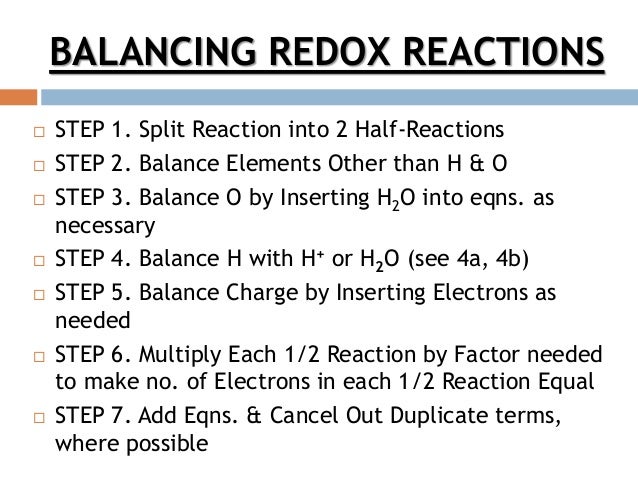
Balancing Redox Reactions
6 Reduction of Cu2+ by Aluminum. Oxidation is the loss .Write and balance the redox reaction that has silver ions and aluminum metal as reactants and silver metal and aluminum ions as products. Redox Reactions as The Basis For Titrations. Earth’s atmosphere contains about 20% molecular oxygen, O 2, a chemically reactive gas that plays an essential role in the metabolism of aerobic organisms and in many environmental processes that shape the world.5 End-of-Chapter Material.Recognize a reaction as an oxidation-reduction reaction.4: Redox Reactions.Oxidation-reduction (redox) reactions involve the transfer of electrons from one atom to another.Acidified Cr 2 O 72- ions can be reduced to Cr 3+ (aq) ions by the addition of zinc.
- How To Make A Linktree On Instagram
- Best Bench Press Angle According To Science
- Zusammen.At Test 2024 _ Kann man sich an mehreren Universitäten anmelden?
- Wrestlemania 37: 5 Theories On Alexa Bliss And The Fiend
- Mba News From The Bay Area: Haas, Stanford
- Besetztzeichen Obwohl Keiner Telefoniert: Woran Liegt Das?
- Engelszahl 2255: Deutungen, Symbolik Und Verborgene Bedeutung
- Maturing Your Terraform Workflow
- The Challenges And Opportunities Facing Gearbox Manufacturing
- Kurzkennzeichen Ohne Fahrzeugbrief/Fahrzeugschein?