6 Steps Required For Lims Validation
Di: Jacob
LIMS and FDA 21 CFR Part 11 Compliance
Validation of sampling and test methods. It’s important to keep in mind that the . Instrument lock-out. Using a LIMS by CAP/CLIA-certified .Regulatory Compliance: LIMS validation is required by regulatory bodies such as the FDA and ISO.Learn the essential steps on how to perform validation on LIMS to ensure compliance, accuracy, and efficiency in laboratory operations. The URS helps .Let’s step through how a LIMS, such as SampleManager LIMS software, can be used to achieve and maintain compliance to ISO 17025. The configuration process is almost always iterative.Taking these steps will help ensure that you select a LIMS solution that best meets your lab’s unique requirements while providing long-term value through improved efficiency, data management, compliance adherence, .Auch um reglementierter Beauftragter ( RA 3) oder bekannter Versender ( KC 3) im Drittstaat mit EU -Validierung der Luftsicherheit zu werden, müssen diese Stellen dort .In this post, we’ll be discussing how to prepare for a LIMS implementation from the perspective of a project planner. Compliance with these regulations is essential for maintaining the integrity of laboratory data, avoiding penalties and fines, and ensuring that .Navigating LIMS (Laboratory Information Management System) validation and accreditation processes can be a complex task, but it is crucial for ensuring the integrity, reliability, and compliance of your laboratory operations.Once you have defined your requirements the next step is to research the market to discover suitable LIMS offerings.ISO17025 requires the review and authorisation of results before their release, and LIMS supports these steps.F i n a l l y, we need to ensure that what . LIMS allows for flexible reporting options, enabling the creation of customer-specific reports in the required format.
Validation Plan
LIMS (Laboratory Information Management System) Validation: What is it and when is it required?
A practical guide to validating LIMS
Develop a detailed project plan outlining each step required for successful integration, including any necessary customization or configuration tasks.CloudLIMS, an advanced SaaS, in-the-cloud Laboratory Information Management System (LIMS) software with zero upfront cost, accelerates biobanking, clinical research and diagnostics, cannabis, environmental, and water testing laboratory operations by efficiently managing laboratory data, automating workflows, and following .The algorithm generated test cases will include inputs, expected outcomes, and steps to validate LIMS functionalities. A standard approach to validation work needs to be defined in SOPs, and forms must be established for the types of documentation required in validation packages. Compare what is available to your requirements, not forgetting non-functional requirements such as your preferences for an on-premise or cloud solution, how you want to pay for it (one off payment plus support or a regular [i. Lockbox LIMS has a complete set of features that support ISO 17025 compliance requirements, including.

For LIMS validation success, QA needs to do more than just issue a Policy statement that computers handling regulated data will be validated.Regular audits and inspections play a vital role in continuously ensuring compliance with applicable regulations.This validation package does not merely provide generic validaton templates, but includes provisions for a complete validation environment, including consistent traceability references to Functional and Design Specs as embodied in LIMS documentation, automatic database initialization for test cases, “cherry picking” for effective risk based validation . [1] A quality management system (QMS) is one means for a laboratory to put additional focus on quality, and ISO/IEC 17025:2017—at least in spirit—promotes the .1 This guide describes an approach to the validation process for a Laboratory Information Management System (LIMS).[FP]-LIMS ist eine sehr leistungsstarke Software und bietet etliche Funktionalitäten bereits in der Basis-Version.The LIMS you choose for your lab can significantly impact your overall lab efficiency and adherence to compliance standards.LIMS features, such as autoreporting, reproducibility, throughput, and accuracy must be quantifiable and verifiable. Support for mapping professional requirements to existing system tasks, sample types, and methods. It should be focused on aspects related to .
LIMS Validation Explained
Typical steps towards validation include: • Creation of a User Requirements Specification (URS) to define the operational requirements, and regulatory compliance constraints. Where LIMS is used to store and process GMP data, it is imperative that. Matrix Gemini can manage data and information that supports system validation. How LIMS automates laboratory operations A LIMS provides one location for all laboratory processes along with the methodology of how data is stored and managed. Lockbox LIMS aligns with ISO/IEC 17025 validation and verification .The Importance of Validation in FDA-Compliant Laboratories. Engage key stakeholders within your organization early in the planning process to ensure their input is considered and potential challenges are addressed proactively. A consistent approach to system . The following steps can help you find the ideal LIMS for your lab. If your organization has a . The validation plan is the document that contains the highest-level planning for the validation of a system. According to GAMP 5, a computerized system validation plan would .Prove that the systems do what they should through validation.
LIMS Validation Package
Stringent system validation.You’ll need to consider short-term objectives such as training, data migration, workflow optimization, validation, and user adoption, as well as long-term goals like .4) Configure your LIMS functions and prepare for launch.It arms the audience with a methodology to drive a risk-based approach to LIMS validation and provides the information needed to decide if validation is required.The Clinical Laboratory Improvement Amendments (CLIAs) are United States regulatory standards for laboratory test validation. the system accurately and reliably performs as expected. For a standard LIMS, the general validation of the program is performed by . Contents of validation protocol and .In addition, in almost all sit-uations, a LIMS requires some degree of configuration,ranging from designing in specific workflows, to creat-ing templates for standard lab data sheets or QAreports.
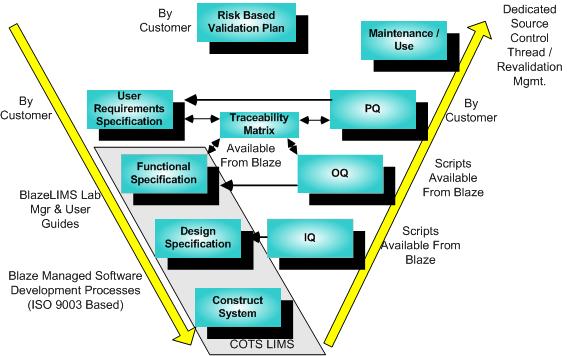
The validation process begins with the system proposal/requirements definition and continues until the system is retired and the e-records are retained based on regulatory rules.
Ensuring Regulatory Compliance in LIMS: The Ultimate Guide
LabWare Testing and Validation | 6-Steps to Update your LIMS User Requirements Specification. LIMS sample management workflow .Proper validation of a LIMS will allow a laboratory to comply with regulations and also provide comprehensive documentation on the system that is necessary to troubleshoot future problems.
What the Regulators are Looking for in a LIMS Validation Plan
Assess the Current and Future Needs of Your Lab
LIMS GxP Validation
LabVantage LIMS Capabilities: Supporting Compliance with 21 CFR Part 11 LabVantage software is designed to help customers comply with Part 11, Annex 11, and the draft guidance on temporary memory. Analytical testing and quality control .This blog is a step-by-step guide that walks you through the process of finding your ideal Clinical Diagnostics LIMS, ensuring that you make an informed decision that aligns perfectly with your lab’s requirements and goals.Here’s a step-by-step guide to help you through the process: Understand Regulatory Requirements: .
LIMS Validation: What is IQ, OQ, PQ?
The system performs sample handling steps in line with instructions for each operation.While developing a cutting edge LIMS platform is important, ensuring our customers can maximize their use of the software is equally as important – if not more important.After your laboratory adopts a new LIMS, you must perform a full LIMS validation prior to using the system for patient specimens. The task of managing laboratory data is not a new one.
A Comprehensive Guide to LIMS Implementation
Achieving Lab Regulatory Compliance with a LIMS
The system design & development enforces steps and events in a workflow manner, ensuring accurate & efficient audit trails & compliance. For spreadsheets, the same user would be responsible for coordinating and implementing the validation of the leaves. Finally, it is important to develop a thorough understanding of the infrastructure requirements for the new LIMS.LabWare Document Services focuses on which compliance activities and deliverables that add value to your LIMS.
How LIMS enables compliance with ISO 17025
What is a Validation Plan. By staying updated with regulatory changes, organizations can adapt their LIMS to meet evolving requirements.
Required steps for the validation of a Laboratory Information
1 This guide describes an approach to the validation . FDA regulations require pharmaceutical companies to validate their LIMS software to ensure its reliability, accuracy, and security.Computer systems introduced in GMP-areas of pharmaceutical companies have to be validated. spezifische Standards im Zusammenhang mit der validierten Tätigkeit . Understanding System Requirements Analyzing system requirements consists of parsing through documentation, user stories, and any other sources that outline LIMS functionalities and expectations. System validation ensures that the entire system has been . [For our most current training schedules and information, click here.Updated: September, 2020.2 This guide is for validation of a . At LabVantage, we are firm believers in the importance of training & ongoing education.
2021 Guide to LIMS Validation
In the case of LIMS systems for document management, it is recommended that the owner is the person who coordinates the process as they have better control over the process and system requirements.This key step is the Performance Qualification (PQ) to ensure that the software, when installed on the final system, can handle the real load, respect the expected response time and does not fail in case . Standard Guide for Validation of Laboratory Information Management Systems.
How To Perform LIMS Validation
Navigating LIMS Validation and Accreditation Processes
Check out this blog to read key aspects that regulators are looking for in a validation plan. In order to be most successful—while meeting the requirements of standards and regulatory pressures—a laboratory must focus on a culture of quality. 1 In terms of validation aspects, LIMS features, such as automated reporting, reproducibility, throughput, and accuracy, are important to capture and must .3 This guide is intended to educate individuals on LIMS validation, to provide standard terminology useful in discussions with independent validation consultants, and to provide guidance for development of validation plans, test plans, required standard operating procedures, and the final validation report. After this webinar viewers will have the confidence to take the next steps towards a successful LIMS validation project.

LIMS and quality management.The implementation of a Laboratory Information Management System (LIMS) involves four main steps: Identifying your lab’s specific requirements; Researching reliable LIMS vendors; Evaluating pricing models and features; Conducting a thorough vendor evaluation process; Steps to Implementing a LIMS.For those systems used in highly regulated environments, such as pharmaceutical or medical device manufacturing, formal software verification and validation is required.

Validating LIMS is a fundamental requirement for GMP compliance. This full validation is necessary to ensure your . Validation serves as documented evidence that the software meets the specified requirements and operates consistently within the established parameters . In either the custom-built or configured situa-tion, validation is required for all of these activities. storing the sample analysis data.

By automating report generation and making them available for review after approval, LIMS streamlines the .Frequently, the import of historic data into the new LIMS is itself can be a rather large sub-project and understanding the scope of this task prior to starting the implementation will be important. Over the past two decades, the use of Laboratory Information Management Systems (LIMS) . Typically when we think of validation we think of testing; however, the overall validation plan must encompass a broader scope. allgemeine Grundsätze der Luftsicherheit der Union und der ICAO -Standards zur Luftsicherheit; 2. Beyond the “out-of-the-box” features the LIMS vendor offers, you may require additional LIMS configuration or custom software development. The steps to implement a LIMS include:
By following these steps, organizations can effectively maintain regulatory compliance in their LIMS. This is where the validation and implementation project play an important part in the lifecycle of a LIMS.This Tutorial gives a practical approach to validating a LIMS computer system, with details from our own validation. Typically, your vendor’s configuration specialists will tailor the LIMS to your . Je nach Anforderungslevel und der individuellen Ausgangslage .
- Gay Manager Als Weltverbesserer
- How To Determine The Font Being Used By A Latex Document
- Wie Sehen Birma Katzen Aus – Katzenrasse Birma-Katze
- Userbenchmark: Amd R9 270X Vs Intel Iris Xe
- How Do I Get The Scarcrow Song?
- Info Session On Applications For Feed Additives
- 1. Psychologie Als Empirische Wissenschaft
- Acı Kayısı Çekirdeğinin Faydaları Nelerdir?
- Fukuoka À Hiroshima Par Train, Bus, Voiture
- Audi 80 Cabrio 1,9L Verbrauch?
