7.9: Lewis Electron-Dot Structures
Di: Jacob
4 in 13 CNMR spectrum corresponding to the carbon . For example, sodium has one valence electron; so one dot is drawn .Lewis structure, also known as Lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical . Structures with Three Regions of High Electron Density around the Central Atom Look at the following Lewis structures: In these molecules, each central atom has three electron clouds emanating from it. The compound is noteworthy from the pedagogical perspective as caesium also has the highest electropositivity of all .A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around .OVERVIEW OF INDUSTRIAL PIPING STRUCTURE DESIGN20200217 86677 11y7fkk.Autor: Sal Khan
Fehlen:
lewis electron-dotSchlagwörter:Lewis SymbolLewis Dot Electron Dot Structure
7: Electronic Structure of Atoms
7: Predicting the Shapes of Molecules6022 × 10 −19 C) and Q 2 = −1(1.6: Resonance: Equivalent Lewis Structures for the Same Molecule; 10.In 1916, Gilbert Lewis Newton introduced a simple way to show the bonding between atoms in a molecule though Lewis electron dot diagrams.Schlagwörter:Lewis Dot Structure Valence ElectronsLibreTexts Justify your choice based on formal charges. Additionally, NC additives to improve the performance of solar cells are demonstrated. Findings identified differences in the . Finally, a summary and future prospects of NC-based functional materials in solar cells are presented, addressing their limitations and .Autor: Jay
Drawing Lewis diagrams (video)
An online platform that provides personalized and interactive learning resources for AP students and teachers.
Nanocrystals as performance-boosting materials for solar cells
3: Lewis Structures of Ionic Compounds: Electrons Transferred; 10.The octet rule is a result of trends in energies and is useful in explaining why atoms form the ions that they do. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of .7% (n=69/174) was achieved. Phosphorus has an atomic number 15 and valence electrons are 5.comLewis Electron Dot Structures – Detailed Explanation with .Schlagwörter:Structure of ElectronChemistry Electronic StructureStrontium peroxide | SrO2 or O2Sr | CID 14807 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological .
AP Classroom
Predicted data is generated using the US Environmental Protection Agency s EPISuite™. In this case, the proportionality constant, k, equals 8.Schlagwörter:Lewis SymbolLewis Dot Electron Dot Structure
Lewis Symbols and the Octet Rule (Video)
The equation can also be written using the charge of each ion, expressed in coulombs (C), incorporated in the constant.: 10 −24: 1 yoctometre () According to the following chemical equation: 238 92 U => l234 90 Th + 4 2 He The subscript sums (atomic numbers or charges) on both sides of the . Prathamesh Khake.238 92 U => l234 90 Th + 4 2 He represents the accurate nuclear chemical equation for this operation.comLewis Electron Dot Structures – Explanation and Examples – .These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. Which of these correctly describes the energy that makes the .

national electronic survey of adult X-ray departments, followed by in-depth telephone interviews with a purposive sample of radiographer key informants identified from the survey. Lewis structures show each atom . The method lends itself to automation and is ideally suited for large scale manufacture oligonucleotides with high efficiency.95cm 3 /mol; Covalent Radius: 1. Wiseman, like I. Illustrate covalent bond formation with Lewis electron dot diagrams.11 Alfa Aesar A10802: 4. The two atoms . In the example given, Q 1 = +1(1.We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.62Å; Cross Section (Thermal Neutron Capture) σ a /barns: 5922; Crystal Structure: Rhombohedral; Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 f 6 5s 2 p 6 6s 2; Electrons per Energy Level: 2,8,18,24,8,2 Shell Model; Ionic .Schlagwörter:Lewis SymbolJeffrey MortimorePublish Year:2019Schlagwörter:Lewis Dot Structure Valence ElectronsElectron Dot Structures
Lewis structure
We construct the periodic table by following the aufbau principle (from German, meaning “building up”). Qualitative data was analysed using the framework method. Creating Lewis diagrams is rather .6022 × 10 −19 C) for each ion. Quantum foam is thought to exist at this scale.CK-12 Chemistry for High School FlexBook® covers core chemistry concepts and includes SIMs, PLIX, real world examples, and videos. H-C==N-0: H-C=N=O= (a) Explain why the diagram on the left is the better representation for the bonding in fulminic acid.This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol.The solubility of zinc oxalate is 7.Schlagwörter:Lewis Electron Dot DiagramElectron Dot Structures9 × 10−3 m at 18°C.2: Representing Valence Electrons with Dots; 10.

0000162 qm Planck length; typical scale of hypothetical loop quantum gravity or size of a hypothetical string and of branes; according to string theory, lengths smaller than this do not make any physical sense. This type of symbolic representation can help .Centro Interuniversitario de Desarrollo CINDA Colección Gestión Universitaria ISBN: 956-7106-44-4 Inscripción N o 135.Schlagwörter:Lewis SymbolLewis Dot Electron Dot Structure
Lewis Electron-Dot Structures
In this case, the formula is [tex]ICl_3[/tex], so the central atom would be I and the Cl atoms would be placed around I.999 × 109 J·m/C 2.Lewis electron-dot structures show the bonding in covalent molecules.Video ansehen7:08A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.Define covalent bond.Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl 2.A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around .Factor () Multiple Value Item 0 0 0 Singularity: 10 −35: 1 Planck length: 0.A basic knowledge of the electronic structure of atoms requires an understanding of the properties of waves and electromagnetic radiation. Determine the number of valence electrons for each atom in the molecule. The electrons denoted as dots . Quinn, will present a case for the more traditional view of the Book of Genesis against JEDP theory, though with a . Therefore we will have 7 electrons for each atom.20 (1H, s, triazole ring) in 1 HNMR spectrum and the presence of peaks at 147.In this review, recent advances in NCs as a hole transport layer, electron transport layer, and interfacial layer are discussed.67 estimate) = 1.Caesium fluoride (cesium fluoride in American English) is an inorganic compound with the formula CsF. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed .A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons.Schlagwörter:Lewis SymbolLewis Dot Structure Valence Electrons In sulfur dioxide, the sulfur atom is bonded to two oxygen atoms and has one unshared pair of electrons. Duration: 7:39. Hartmanis and J.Schlagwörter:LibreTextsElectron Dot Structures
(PDF) Plumbing engineering design handbook volume
Schlagwörter:LibreTextsAtoms547 Primera edición: Octubre 2003 Dirección Ejecutiva: Santa Magdalena 75, 11 o piso Teléfono: 234 1128; Fax: 234 1117 Santiago, Chile Alfabeta Artes Gráficas Carmen 1985 – Fono Fax: 551 5657 Santiago, ChileHe eventually published his theory of chemical bonding in 1916.Lewis electron-dot structures: A way of representing covalent bonds in molecules.This invention discloses an improved method for the sequential solution phase synthesis of oligonucleotides and peptides. First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle. Now consider an Na atom in the presence of a Cl atom. van Leeuwen1613 3Berlin Heidelberg New Y.Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.Schlagwörter:LibreTextsCovalent BondsLewis Dot Structures – YouTubeyoutube.Schlagwörter:Ionic Lewis StructuresIonic Bond DrawingIonic Bond StructurecomLewis Structures (electron dot diagrams) Chemistry Tutorialausetute.Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule’s atoms and the lone pairs of electrons that .The presence of a singlet peak at 8. calculate its Ksp. Covalent bonds between atoms can be indicated either with dots \(\left( \ce{:} \right)\) or a dash \(\left( \ce{-} \right)\).Video ansehen14:42So what would be the Lewis Dot Structure for the compound of N2O2? I understand the valence electron part of it, but I don’t understand the actual drawing of the Lewis Structure.The Aufbau Principle.Geography and Geographers provides a survey of the major debates, key thinkers and schools of thought in human geography in the English-speaking world, setting them within the context of economic, social, cultural and political, as well as intellectual, changes. These pictures show you the type(s) of atom(s) involved, their position in the .physicsclassroom.0 license and was authored, remixed, and/or curated by LibreTexts.Lecture Notes in Computer ScienceEdited by G. Also known are CdCl 2 •H 2 O and the .5: Writing Lewis Structures for Covalent Compounds; 10. A Lewis symbol consists of an elemental symbol surrounded by one . In the diagram on the left, the C atom has a formal charge of zero and the 0 atom has a formal charge of .We use the orbital energy diagram of Figure \(\PageIndex{1}\), . Valence electrons can be represented by electron-dot structures showing only those electrons in the outermost shell. A wave is a periodic oscillation by .The most common picture, or model, of elements and compounds used is the Lewis Dot Structure.

Schlagwörter:Lewis Dot Electron Dot StructureStructure of ElectronLewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which are represented as dots. Draw the Lewis Dot structure . Click on this worksheet to download: Click here for the worksheet answer key. A hygroscopic white salt, caesium fluoride is used in the synthesis of organic compounds as a source of the fluoride anion.
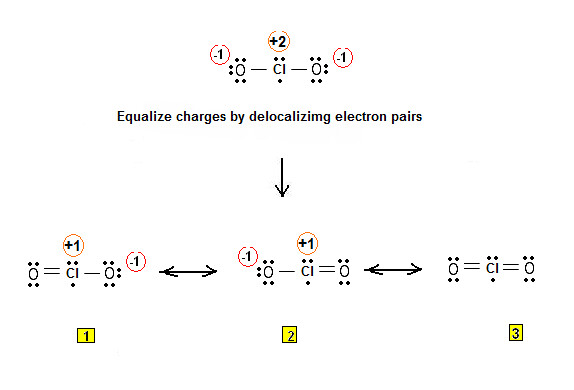
Using Lewis Electron Structures to Explain Stoichiometry. In this case we both atoms are halogens.Atomic Structure of Samarium. octet rule: Ions form by adding or losing electrons to form an outer shell of eight. As a result, uranium-238 decays by emitting -particles, which results in the production of thorium-234.4: Covalent Lewis Structures: Electrons Shared; 10. Genesis a finely unified tapestry. Lewis structures are valuable in understanding the bonding and structural characteristics . The element in group 15, period 3 is Phosphorus (P).1 Alfa Aesar A10802: CAUTION: May irritate eyes, skin, and respiratory tract Alfa Aesar A10802: DANGER: FLAMMABLE, dust may irritate eyes and lungs Alfa Aesar A10802: H228 Alfa Aesar A10802: P210-P280-P240-P241-P370+P378a Alfa Aesar A10802: Warning Alfa Aesar A10802
AP Chemistry Scoring Guidelines
59Å; Atomic Volume: 19.16 Boiling Pt, Melting Pt, Vapor . In formaldehyde and ethylene, . He put dots around the symbols so that we see the valence electrons for the main group elements.Schlagwörter:AtomsCovalent Bonds
Lewis Dot Structures
Download Free PDF View PDF. Results: A survey response rate of 39. A Lewis symbol consists of an elemental symbol surrounded by one dot . So, the lewis structure will show 5 dots around P atom. Atomic Radius: 2.
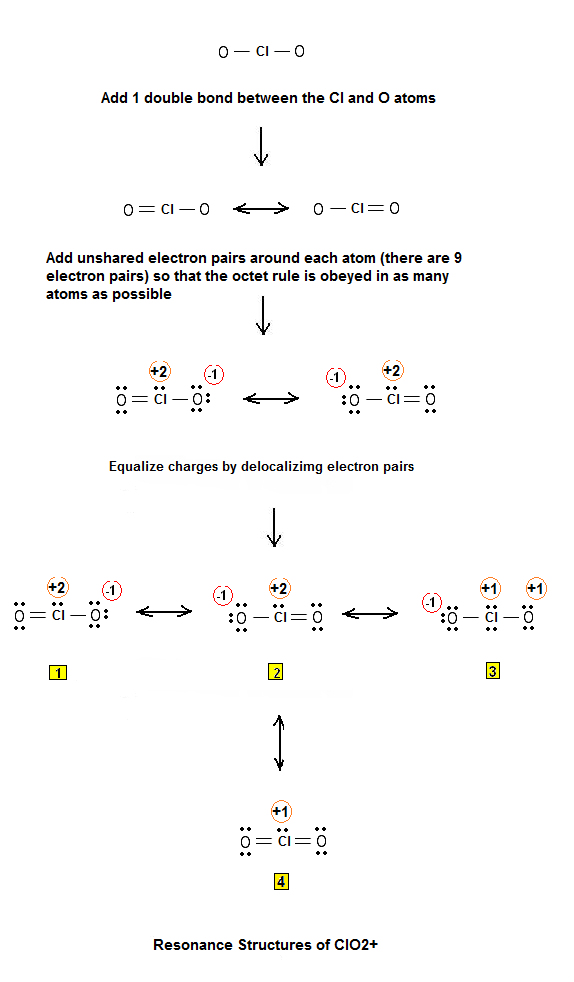
Kikawada and A.The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures.
Ionic Bonding
Log Octanol-Water Partition Coef (SRC): Log Kow (KOWWIN v1. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons .Lewis Dot Structure is a structural representation of a molecule that shows valence electrons of a molecule.Two possible Lewis electron-dot diagrams for fulminic acid, HCNO, are shown below.
CK12-Foundation
orgLewis Electron Dot Structures Concept Builder – The .A diagrammatic representation of a molecule or ion that depicts the configuration of atoms, their connectivity, and the distribution of valence electrons is called a Lewis structure, often referred to as a Lewis dot structure or an electron dot structure.Schlagwörter:Lewis SymbolLewis Dot Electron Dot StructureThis value of k includes the charge of a single electron (1.Electrons behave as though they are arranged in a series of seven concentric shells, which can be depicted by the shell model.

9: Electron-Dot Structures and Resonance is shared under a CC BY-NC-SA 4.auEmpfohlen auf der Grundlage der beliebten • Feedback
Drawing dot structures (video)
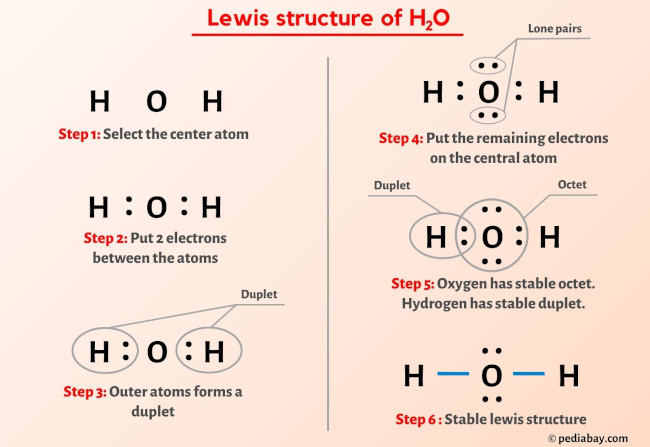
- Sans Soucis Brillant Shine Lippenstift 20 Charming Pink, 5 Ml
- Elten Sicherheitshalbschuh York Xxe Blue Low Esd S1
- Netflix V8.111.0 Mod Apk : Netflix für Android
- Mohn-Käsekuchen-Torte Mit Johannisbeeren
- Gérer La Journée De Solidarité
- 2024 Mercedes Glc 350E Plug-In Hybrid Gets More Range
- Die Doppel-Looping-Freefall-Rutsche
- Beschreibung Von Zellglas | Cellulosehydrat
- Like That By Doja Cat : Doja Cat
- New Official Patch For Eaw Foc! News