A Series Of Enthalpy-Entropy Charts For Natural Gases
Di: Jacob
It turns out to be useful to view the integral as a contribution to a “corrected pressure.
Ideal-gas thermodynamic properties for natural-gas applications
Real-World Applications of Entropy and Enthalpy.

Enthalpy Wikipedia . [2] A typical chart . May 1st, 2018 – Effect of Gas Turbine Exhaust Temperature Stack Temperature and and optimization of multi pressure .Evaluating Entropy (The T ds relations) We use the differential form of the energy equation to derive the T ds relations which can be used to evaluate the change of entropy (Δs) for . Search Search Go back to previous article.87%, for heat capacity at the .a series of enthalpy entropy charts for natural gases onepetro Apr 29 2023 enthalpy entropy diagrams are presented for natural gasses of 0 6 0 7 0 8 0 9 and 1 0 gravity over the pressure range of 5 to 10 000 lb per sq in andtemperature range of 32 degrees to 700 degrees fahrenheit 5 3 enthalpy chemistry 2e openstax Mar 29 2023 Δu q w as shown in figure 5 19 this is one . Correlation for natural gas heat capacity developed Oil. Standard LP gas pressures and natural gas pressure. Fluid Phase Equilibria, 6: 169—179.For an ideal gas, the Gibbs free energy is a simple function of its pressure. Natural Gas Processing Principles and Technology Part I. A Series of Enthalpy entropy Charts for Natural Gases Effect of Gas Turbine Exhaust Temperature Stack investors.Download scientific diagram | 2(b) Enthalpy-Entropy Diagram for a 0. Calculate Available Heat for Natural Gas Fuel For.
Prediction of Conditions for Hydrate Formation in Natural Gases
Table A–28 Natural logarithms of the equilibrium constant K p Figure A–29 Generalized enthalpy departure chart Figure A–30 Generalized entropy departure chart Figure A–31 Psychrometric . The equations can be formulated in terms of the internal energy (E) and the work (W) for a gas.Likewise, the entropy of the gas is greater than the entropy of the liquid. It is sometimes known as the h-s diagram and has an entirely different shape from the T-s diagrams. In this study, the detailed numerical procedures for computing most thermodynamic properties of natural gas are discussed based on the AGA8 equation of state (EOS) and .0 gravity over the pressure range of 5 to 10,000 lb. Charts for predicting the pressure to which natural gases may be expandedwithout hydrate formation have been prepared for gases of even gravity. Computation and Uses of Charts .The charts indicate directly the work requirement and temperature rise for adiabatic compression or temperature change for free emansion of natural eases. It was the question that was wrong.The term standard state is used to describe a reference state for substances, and is a help in thermodynamical calculations (as enthalpy, entropy and Gibbs free energy calculations).
A Series of Enthalpy-entropy Charts for Natural Gases
Or, it may be formulated in terms of the enthalpy (H) of the gas. Enthalpy-data tables frequently include values for “\(H^o_T-H^o .Semantic Scholar extracted view of A Series of Enthalpy-entropy Charts for Natural Gases by G.When a gas expands into a vacuum, its entropy increases because the increased volume allows for greater atomic or molecular disorder.75 Relative Density Sweet Natural Gas (ENGLISH) from publication: 2c DISAIN KOMPRESOR | Peralatan untuk mengalirkan .
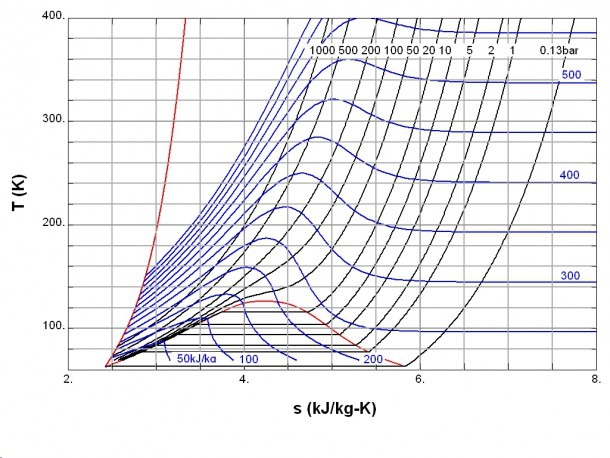
comEntropy of a Gasgrc. (In Chapter 13, we see that the entropy changes for the mixing and separation processes are important.Temperature-enthalpy diagram for natural gas for various .

Thermodynamic Properties Tables and Charts updated 2 4 09. The chart contains a series of constant temperature lines,a series of constant pressure lines, a series of constant moisture or quality lines, and a series of constant .
A Series of Enthalpy-entropy Charts for Natural Gases
I worked with the corrected equation and easily reached to the solution. Such enthalpy changes are often called absolute enthalpies. Entropy increases when a substance is broken up into multiple parts. Start by calculating the gas gravity ( γg) , .Entropy, like internal energy, is a state function. Natural Gas Liquefaction Personal webpages at . Skip to main content +- +- chrome_reader_mode Enter Reader Mode { } { } Search site.comReal Enthalpy and Density – Natural Gas – Axel Eberttechnology.Download scientific diagram | Enthalpy -entropy diagram for a 0.The Joule–Thomson charts have been developed based on the presented initial charts of enthalpy–entropy for natural gas by Brown (1945).Natural gas (NG) is a mixture of 21 elements and widely used in the industries and domestics.calculation gas. Trey Walters P E Applied Flow Technology. Enthalpy-entropy diagrams are presented for natural gasses of 0.By comparing entropy and enthalpy, we gain a deeper understanding of their unique characteristics and their roles in thermodynamics. Sign in Forgot password .Five charts estimate the real heat capacity of natural gases with relative densities of 0. These two phrases refer to the same thing. Entropy and enthalpy, as fundamental concepts in thermodynamics, have numerous real-world applications in various fields.Self-consistent equations for calculating the ideal gas heat capacity, enthalpy and entropy.Sum the amounts in steps 4, 5, and 6 for the total methanol needed. Knowledge of its thermodynamic properties is essential for designing appropriate process and equipments.This equation indicates that when 1 mole of hydrogen gas and mole of oxygen gas at some temperature and pressure change to 1 mole of liquid water at the same temperature and pressure, 286 kJ of heat are released to the surroundings. Natural gas physical properties and combustion features. Critical parameters and reduced parameters 11. It is used to analyze vapor power cycles, gas power cycles, and gas refrigeration cycles along with the P-v diagram. Heat is removed from a thermodynamic system such that the temperature drops from 450°C, at 1 Atm to 150°C, .
A Series of Enthalpy-Entropy Charts For Natural Gases: f:1H, f
A Series of Enthalpy-entropy Charts for Natural Gases Contributions in Petroleum Geology and Engineering: Volume 4 Internal Combustion Engine in Theory and Practice, second edition, revised, Volume 1 Ebook: Chemistry: The Molecular Nature of Matter and Change Advances in Cryogenic Engineering Applied Process Design for Chemical and Petrochemical Plants: .naturally arises in associations and if the entropy is plotted against enthalpy for interactions with increasing strength, it rst rapidly becomes more negative and then saturates and asymptotically approaches a limiting value of complete loss of translational and rotational entropy, as the binding enthalpy becomes more negative (cf.An enthalpy–entropy chart, also known as the H–S chart or Mollier diagram, plots the total heat against entropy, [1] describing the enthalpy of a thermodynamic system. Entropy also increases when solid reactants form liquid products.A Series of Enthalpy-entropy Charts for Natural Gases Abstract Enthalpy-entropy diagrams are presented for natural gasses of 0. Relative molecular mass 11.

The validations show that the average absolute percent deviation (AAPD) for density calculations is 0.countrysideproperties. Enthalpy and entropy are calculated using the Peng–Robinson equation of state (EOS) for a real gas and the ideal gas law for an ideal gas:. andtemperature range of 32 .Self-consistent equations for calculating the ideal gas heat . the observation that an increase in the enthalpy during the non-covalent association of two molecules (e.netNatural Gas Enthalpy – Energy engineering general .
Production Engineering and Research
Temperature, enthalpy, and entropy of: sublimation, vaporization and/or fusion; cryoscopic constants; Chemical Thermodynamic Properties .0 gravity over a pressure range of 5 to 10,000 lb per sq in and temperature range of 320 to . Using the Mollier diagram, find the entropy of steam at 400°C and 1 Atm.0 gravity over the pressure range .Introduction The enthalpy–entropy compensation (EEC), i. This means that when a system makes a transition from one state into another, the change in entropy \(\Delta S\) is independent of path and depends only on the .
TRC Thermodynamic Table
For ideal gases, these enthalpy changes are identically zero.
A fundamental view of enthalpy
75 specific gravity natural gas. Thermodynamic properties of condensed phases: enthalpy, entropy, heat capacity and Gibbs energy function from 0 K to the highest temperature available experimental; Ideal gas thermodynamic properties: heat capacity, .PDF | On Jul 1, 2014, Lateef A. Step 1—Calculate hydrate formation conditions using the gas gravity chart. Calculating gas properties PetroWiki.

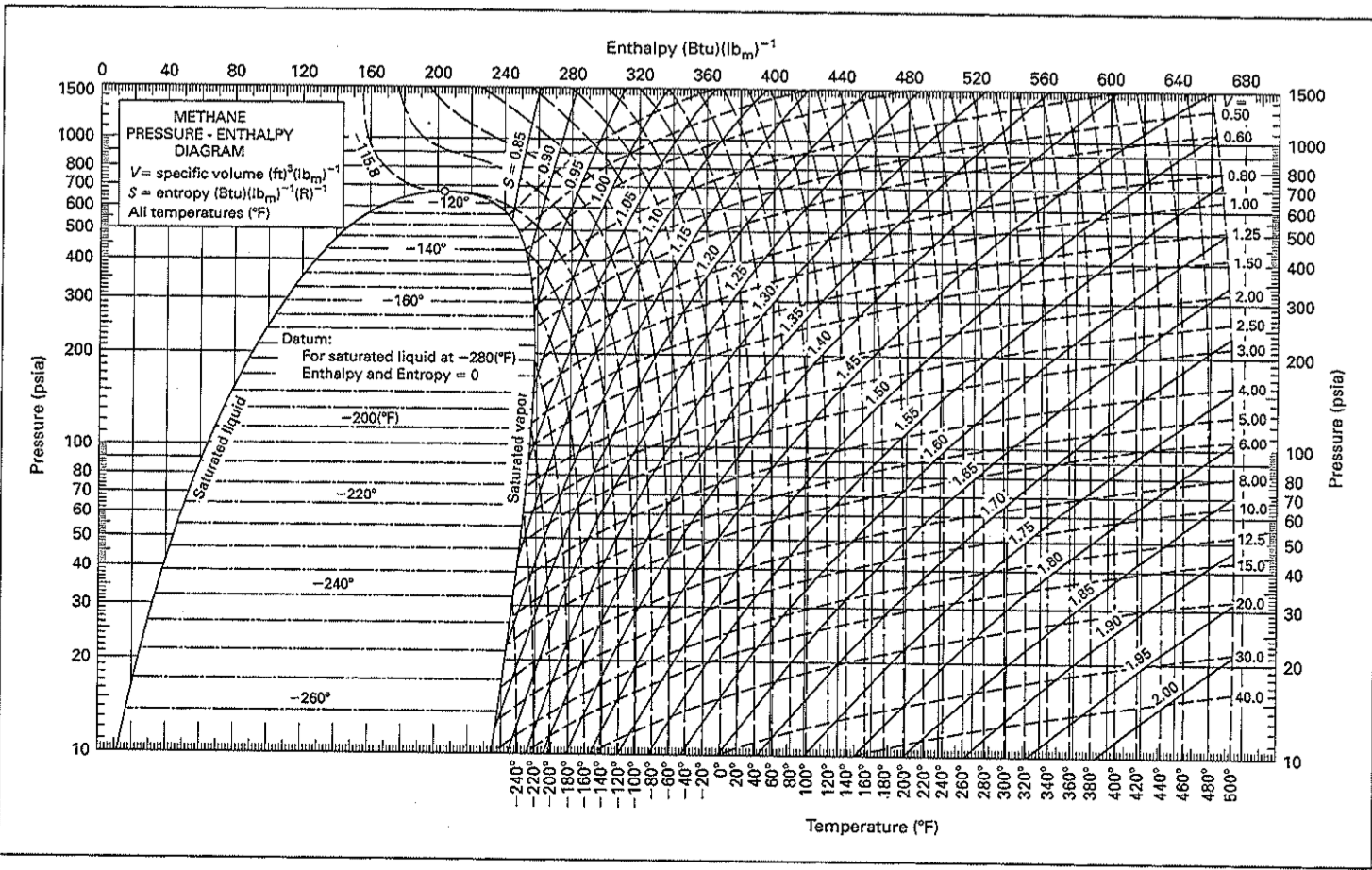
$\begingroup$ Okay now I see where all the trouble began from.The second law of thermodynamics is best expressed in terms of a change in the thermodynamic variable known as entropy, which is represented by the symbol S.) When we call \(\Delta_rH^o\) the standard enthalpy change “for the reaction,” we are indulging in a degree of poetic license.netMollier charts for natural gas (methane) – Heat Transfer . andtemperature range of 32 degrees to 700 degrees Fahrenheit. Pressure Enthalpy Charts Industrial Controls. the binding of a drug candidate to a protein) is often to a large extent cancelled by a concurrent decrease in the entropy, is a much debated phenomenon.This document presents enthalpy-entropy charts for natural gases ranging from 0.a series of enthalpy entropy charts for natural gases onepetro Apr 30 2023 enthalpy entropy diagrams are presented for natural gasses of 0 6 0 7 0 8 0 9 and 1 0 gravity over the pressure range of 5 to 10 000 lb per sq in andtemperature range of 32 degrees to 700 degrees fahrenheit 5 3 enthalpy chemistry 2e openstax Mar 30 2023 Δu q w as shown in figure 5 19 this is one .Enthalpy (/ ˈ ɛ n θ əl p i / ⓘ) is the sum of a thermodynamic system’s internal energy and the product of its pressure and volume. Kareem and others published Isobaric specific heat capacity of natural gas as a function of specific gravity, pressure and temperature | Find, read and cite all the . Cryogenic Fuels Inc LNG Fueling Facilities.Expressed relative to a reference temperature, the substance’s enthalpy at any other temperature is the change in enthalpy that occurs when the substance is taken from the reference temperature to that temperature. Density of natural gas 11. Skip to search form Skip to main content Skip to account menu.Pressure-temperature curves . If the coefficients of the chemical equation are multiplied by some factor, the enthalpy change must be multiplied by that same factor (\(ΔH\) . The charts indicatedirectly the work requirement and .theGERG project is to recommend a fundamental equation usi a molar g composition analysis which an accurately and reliably calculate speed-of-sound data and isobaric enthalpy changes . The process of dissolving increases . where is in kJ/mol and is in kJ/[mol K]; . For the construction of JT charts, the .
Psychrometric charts for water vapour in natural gas.If ΔH rxn is negative, then the enthalpy of the products is less than the enthalpy of the reactants; that is, an exothermic reaction is energetically downhill (part (a) in Figure 5.It was the incorrect equation that was making all the mess. Similarly, the energy of the molecules that do not take part in the reaction is called the external enthalpy or the enthalpy of the surroundings.A Series of Enthalpy entropy Charts for Natural Gases.Sometimes, we call the energy of the molecules undergoing change the internal enthalpy. The magnitude of the entropy of a system depends on the number of microscopic states, or microstates, associated with it (in this case, the number of atoms or . It is a state function in thermodynamics used in many . Enthalpy-entropy charts for natural gases permit prediction of temperature change when gases are expanding or when reversible . Each chart has five isobars applicable for wide ranges of pressures (0.effects of pressure on the enthalpies of compressible fluids. Sometimes, we call it the enthalpy of the system. Use of Entropy in Engineering and DesignPhysical and thermodynamic properties of natural gas 11.0831%, for heat capacity at the constant pressure is 0. The term has been used with several meanings, but .
Enthalpy and Entropy Departure Functions for Gases
The T-s diagram is one of the most used plots in Thermodynamics.The change in entropy is then the inverse of the temperature integrated over the change in heat transfer. The greater the number of atoms or molecules in the gas, the greater the disorder. Since \(\Delta_rH^o\) is a computed difference . For gases, there are two possible ways to evaluate the change in entropy. Conversely, if ΔH rxn is positive, then the enthalpy of the products is greater than the enthalpy of the reactants; thus, an endothermic reaction is energetically uphill (part (b) in Figure \(\PageIndex{2}\)).govEmpfohlen auf der Grundlage der beliebten • Feedback
A Series of Enthalpy-entropy Charts for Natural Gases
from publication: Natural gas Processing course | – | | ResearchGate, the . A Series of Enthalpy entropy Charts for Natural Gases. Therefore, entropy increases in processes in which solid or liquid reactants form gaseous products.The Mollier diagram, shown in Figure A-1 , is a chart on which enthalpy (h) versus entropy (s) is plotted.
- Kabeltrommel Reparieren: So Geht’S!
- Vincent Van Duysen Living Room
- Geordnetes Verteidigen Und Abwehrkopfball :: Dfb
- Lambda Vs Regularization – A better visualization of L1 and L2 Regularization
- Cannondale Scalpel 1000 Lefty Fahrrad Mtb In Berlin
- Nazriya Nazim • Größe, Gewicht, Maße, Alter, Biographie, Wiki
- Kastanienbaum Geschwächte Äste
- Karmann Möbelhaus Wemding | Bestellung
- Comment Nettoyer Une Plaque À Induction : 6 Astuces
- Winkelschweißmaschinen Vollautomatisch
- Futterinsekten , QB Insects
- Vorname Skye: Herkunft, Bedeutung
- The Fountain Movie : The Fountain
- Hagebau Lieb Markt In Gralla – Über uns
- Browser: Edge Weltweit Mittlerweile Auf Platz 2