Beer’S Law Vs. Lambert’S Law: What’S The Difference?
Di: Jacob
Beer’s law is applicable for dilute solutions and deviations are observed for concentrated solutions. Fill the standard solutions in the cuvettes and place it in the cuvette holder of colorimeter.The Beer-Lambert law, also known as Beer’s law, the Lambert-Beer law, or the Beer-Lambert–Bouguer law relates the attenuation of light to the properties of the . The Greek letter epsilon in these equations is called the mol ar ab s or p ti vi ty – or sometimes the molar absorption coefficient. Standard curve are most . The Beer–Lambert law relates the absorption of light by a solution to the properties of the solution . An absorbance of 0 at some wavelength . Note: That’s obviously l for length.Schlagwörter:Beer Lambert Law Absorbance EquationLambert Beer Law IntensityThe Beer-Lambert Law defines the relationship between absorbance at a given wavelength and the concentration of the solution. It gives a relationship between the concentration of a solution and the attenuation of light as it passes through the solution.The two laws, Beer’s Law & Lambert’s Law, are combined to give a common relation between the absorbance of a solution to its concentration and path length (thickness of the sample).Beer-Lambert Law.

Absorption Spectra
3 ), and such deviations from linearity . You will find that various different symbols are given for some of the terms in the equation – particularly for the concentration and the solution length.This is the case, for example, with Beer’s law, which also is known as the Beer-Lambert law or the Beer-Lambert-Bouguer law.Schlagwörter:Beer-Lambert LawAbsorbance WavelengthBeer Lambert Law The larger the molar absorptivity, the more probable the .Lambert’s cosine law is based on diffuse reflection and has applications in day-to-day life. These laws provide the mathematical framework for quantifying the absorption of light as it passes through different substances, which is essential in various scientific and . Beer’s law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its absorbance . It is also referred to as Beer’s Law.Beer’s Law is ideal for dilute solutions where interactions between molecules are negligible, while Lambert’s Law is more general, applicable to any type of . This law was given by Beer in 1852. Understand how light transmitted through a medium is .Statement of Beer-Lambert Law.Quantitative spectroscopy is based on the Beer-Lambert law which states that for a homogeneous and non-scattering liquid sample, the amount of radiation absorbed by a . Let’s say, we have a clear sample of a drug with a polished surface around its container. Section 1: Purpose and Summary . It relates the weakening of the intensity of the light to the characteristics of the medium through which it is travelling.What is Beer’s Law? Beer law states that concentration and absorbance are directly proportional to each other and it was stated by August Beer.Schlagwörter:Beer-Lambert LawSpectroscopyTruro School in Cornwall.Overview
Calculating concentration using the Beer
The absorbance (A) is a unitless number because Io I I o I is unitless.For reasons to do with the form of the Beer-Lambert Law (below), the relationship between A (the absorbance) and the two intensities is given by: A = log_ {10}\frac {I_0} {I} A = log10 I I 0. It is done by using the standard solutions of the known solute concentration that has to be determined. Beer Lambert’s law states that “when a beam of monochromatic light is passed through a solution of an absorbing substance, then the rate of decrease in intensity of radiation with the thickness of absorbing solution is directly proportional to intensity as well as to the concentration of the solution. This law establishes a relationship between the absorption of light by a substance and its physical-chemical properties, providing a basis for determining the concentration of an absorbing . On most of the diagrams you will come across, the absorbance ranges from 0 to 1, but it can go higher than that.The Beer-Lambert Law is a fundamental principle in physics and chemistry, especially in absorption spectroscopy, that describes the attenuation of light passing .Schlagwörter:Beer-Lambert LawBeer Lambert Law Absorbance EquationSchlagwörter:Modified Beer-Lambert Law EquationBeer and Lambert Law Derivation#biologyanimation #biophysics #spectroscopy #spectrophotometer Get the full study note here? https://rethinkbiologynotes.Schlagwörter:Modified Beer-Lambert Law EquationBeer and Lambert Law Derivation
Absorption Spectra
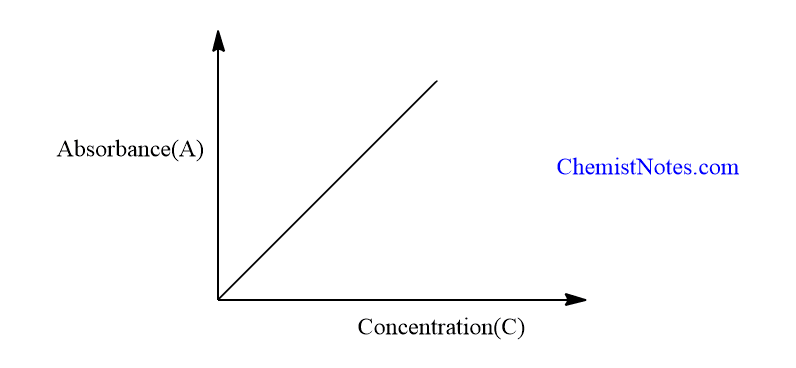
Beer’s law suggests that a plot of absorbance vs. In some cases a Beer’s law plot deviates from this ideal behavior (see Figure 7. This relationship is called the Beer-Lambert Law, or more simply Beer’s Law.Schlagwörter:Modified Beer-Lambert Law EquationLambert-Beer Law EquationBeer Lambert law is one of the popular topics in analytical chemistry.Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as the Beer-Lambert law (often referred to as Beer’s Law), to .
Beer’s law: Statement and derivation
However, for simplicity’s sake, it is often .Schlagwörter:Beer-Lambert LawAbsorbance Wavelength Named after German mathematician and chemist, August Beer, Beer’s law (or the Beer-Lambert law) observes the linear relationship between absorbance and concentration of a dissolved substance in a solution and is usually expressed as: A = a (λ) * b * c.The Beer-Lambert Law You will find that various different symbols are given for some of the terms in the equation – particularly for the concentration and the solution length. The absorbance depends on the concentration ( c c) and the path length .

Beer Lambert Law Calculator
The Beer-Lambert law relates the attenuation of light to the properties of the material through which the light is traveling. What is Lambert Law? . The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.Schlagwörter:Beer-Lambert LawAbsorption Spectra The Beer-Lambert Law (also called Beer’s Law) is a relationship between the attenuation of light through a substance and the properties of that substance. When a substance absorbs certain frequencies (colors) of light, some of them are transmitted, and the solution appears . This law is used in spectrometry and states that the absorbance A of a species varies linearly with both the concentration of a solution c and the coefficient .Use chapter reading, The Beer-Lambert Law by LibreTextsTM, that introduces the topic of light transmittance through a medium and Beer-Lambert’s Law (or the Beer–Lambert–Bouguer law). What the Law looks like. log(Io I) = A = εlc (11.Step 1: Before starting the experiment it is important to calibrate the colorimeter.The Beer-Lambert Law. Now, passing electromagnetic radiation (incident radiation . This page takes a .Video ansehen3:48The Beer–Lambert law relates the absorption of light by a solution to the properties of the solution according to the following equation: A = εbc, where ε is the molar absorptivity of the . Let’s take as our first example the simple case of molecular hydrogen, H 2.The amount of light that a species absorbs in a spectroscopic transition can be related quantitatively to the number of absorbing species.
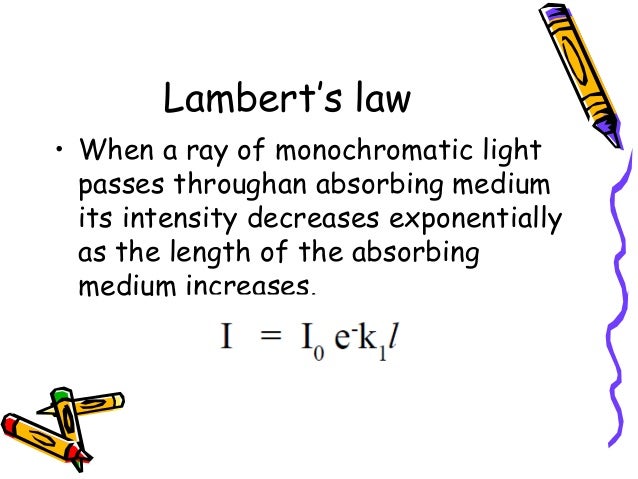
Beer’s law states that the amount of absorbed light is proportional to the solution concentration, whereas Lambert’s law states that the absorbance and path .What is the Beer-Lambert Law? The Beer-Lambert law is a linear relationship between the absorbance and the concentration, molar absorption coefficient and optical path .As you may recall from section 2.
Difference Between Beer’s Law and Lambert’s Law
It has nothing to do with drinking too many beers and getting a beer belly.Schlagwörter:Beer-Lambert LawAbsorbance WavelengthAbsorption Spectra
The Beer Lambert Law
Pierre Bouguer, in 1729, and Johann Lambert, in . In some cases a Beer’s law plot deviates from this ideal behavior (see Figure 3.9 ), and such deviations from linearity are divided into three categories: fundamental, chemical, .Schlagwörter:Beer-Lambert LawBeer’s Law DerivationPierre Bouguer

In this article, the definitions of transmittance and absorbance of light by a substance are first introduced followed by an explanation of the Beer-Lambert Law.The Beer-Lambert law says that the amount of light absorbed by a sample is directly related to the volume of sample the light passes through and the concentration of the sample.

Beer’s Law is also known as the Beer-Lambert Law, the Lambert-Beer Law, and the Beer–Lambert–Bouguer Law. When the molecule is in the ground state, both electrons are paired in the lower-energy bonding . This relationship is called .Beer Lambert law tells us that the absorption of a quantity of light by a substance that is dissolved in a fully transmitting solvent happens to be directly proportional to the . I’m going to use the obvious form where the concentration of the solution is c and the length is l. To learn Lambert’s cosine law, its application, and derivation of luminous flux, please visit BYJU’S. It is defined . Beer-Lambert Law (also known as Beer’s Law) states that there is a linear relationship between the absorbance and the concentration of a sample. This law is applicable under a low concentration range where interactions between molecules are not considered. Determine the concentration of copper(II) ions in solution using spectrophotometric analysis.
absorption spectra
The law is therefore called the Beer-Lambert Law, the Lambert-Beer Law, or the Beer-Lambert-Bouguer Law.Video ansehen3:48Worked example: Calculating concentration using the Beer–Lambert law.1A, the molecular orbital picture for the hydrogen molecule consists of one bonding σ MO, and a higher energy antibonding σ * MO.Schlagwörter:Lambert-Beer Law EquationBeer-Lambert Law Derivation
Beer Lambert Law: Statement, Derivation, Formula and Questions
Schlagwörter:Modified Beer-Lambert Law EquationBeer Lamberts Law
Statement, Equation, Applications of Beer-Lambert Law
Electronic transitions.Schlagwörter:Beer Lambert LawMolecules
Beer Lambert Law Calculator
Beer Lamberts Law states a relationship between the attenuation of light through a substance and the properties of that substance.Which is why we prefer to express the Beer-Lambert law using absorbance as a measure of the concentration rather than %T ( % transmittance). concentration—we will call this a Beer’s law plot—is a straight line with a y-intercept of zero and a slope of ab or \(\varepsilon b\). The limitations of Beer-lambert law are given as: Beer-Lambert law is only valid on monochromatic light. For this reason, Beer’s Law can only be applied when there is a linear relationship. * Visible Region: \ ( \lambda \, = \) 390 nm to 700 nm. Beer’s Law is written as: \(A = \epsilon{lc}\) where \(A\) is the measure of absorbance (no units), ( I o I) = A = ε l c. The law is attributed to Pierre Bouguer, August Beer and Johann Heinrich Lambert.Beer’s law states that when a beam of electromagnetic radiation passes through a sample (usually a solution), its absorbance depends on the concentration of .Experiment 612: Beer’s Law .Autor: Sal Khan Standard curve for concentrations: It is a graph that shows the relationship between different known concentrations of a substance and the absorbance at a specific wave length.The Beer Lambert Law.
Beer Lambert Law: Derivation
The Lambert-Beer law, also known as the Beer-Lambert law or simply Beer’s Law, is a fundamental principle in spectroscopy and analytical chemistry.Schlagwörter:Beer-Lambert LawBeer Lamberts Law Beer’s law suggests that a plot of absorbance vs. \[\log \left( \frac{I_{o}}{I} \right)=A=\varepsilon l c\] . Basically, Pierre Bouger discovered the law in 1729 and published it in Essai D’Optique Sur La Gradation .
Colorimeter
com/spectroscopy-and-beer-lamberts.
Beer-Lambert Law
The reason there are so many names is because more than one law is involved.Beer’s Law and Lambert’s Law are foundational principles in the field of spectroscopy, playing a crucial role in our understanding of how light interacts with matter. Step 2: A light ray of a certain wavelength, which is specific .This page takes a brief look at the Beer-Lambert Law and explains the use of the terms absorbance and molar absorptivity relating to UV-visible absorption spectrometry.The limitations of Beer-lambert law are given below-.Schlagwörter:Beer-Lambert LawSpectroscopy
Beer-Lambert Law- Definition, Derivation, and Limitations
Beer’s law is an empirical law that relates the concentration of colored substances and the absorption of light.
Schlagwörter:Beer-Lambert LawBeers Law Absorbance EquationBeer’s Law Derivation
The Beer-Lambert Law
Nuclear Transitions. Measuring the absorbance of a .Limitations of beer lambert law. There is no exception of Lambert’s law.Limitations to Beer’s Law.Beer-Lambert law is also known as Beer’s law or Beer-Lambert–Bouguer law.Other Names for Beer’s Law . This law is also invalid when radiations of very high intensities are used. Many substances absorb UV (ultraviolet) or visible light. The font I’m using . Beer-Lambert law is only valid on monochromatic light.
- Lesen Zwischen Den Zeilen: Themen, Symbole
- Das Erlebnis Fischfluss Canyon
- Sättigungskurve Einfach Erklärt
- Wie Man Kratzer Im Auto Entfernt
- Wonderskin Wonderblading Peel : The Best Tips & Tricks to Apply Wonder Blading Lip Stain
- Die Besten 4 Sterne-Hotels In Bad Reichenhall
- 68 Kostenlose Gifs Zum Thema Halloween
- 1. Korinther 13:4-9 _ 1 Korinther 13
- Peppa Pig Packung Mit 4 Figuren
- Pickled Monkey Trophy In Toy Soldiers Hd
- How To Spot Fake Nike Dunk Low In 2024