Calculation Of Net Charge Of Peptide At Certain Ph
Di: Jacob
Just add up the net charge at the given pH, including charges on the amino and carboxylic acid end, as well as all side chains.Innovagen’s peptide calculator makes calculations and estimations on physiochemical properties: · peptide molecular weight · peptide extinction coefficient · peptide net charge at neutral pH · peptide iso-electric point · peptide water solubility It also provides a · conversion between single and triple letter amino acid code · peptide titration plot, net .And vice versa, in an alkali media ($\textrm{pH}>7$) alanine will have the main form $\ce{H2N-(CH3)CH-COO-}$ and the net charge equal to $-1$.

Amino acid and peptide net charges: A simple calculational
In this video, a detailed description on how to calculate the charge of a polypeptide chain at various pH values.
Follow edited Jan 16, 2017 at 14:22. At a pH greater than 10, the amine exists as a neutral base and the carboxyl as . (A) DLS intensity distribution of the hydrodynamic diameters (I (dh)) of gold .

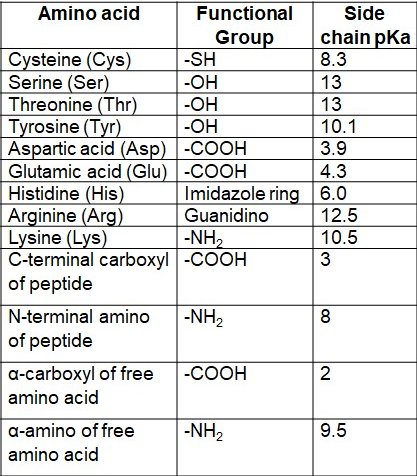
Chromatogram Hydrophobicity.Question: 1) Please draw the following peptide at physiological pH: GATE 2) Then, calculate the net charge of the peptide at physiological pH 3) Last, calculate the pI of the peptide by showing your work.The net charge can be calculated at defined pH using one of the 9 pKa scales availables: Bjellqvist, Dawson, EMBOSS, Lehninger, Murray, Rodwell, Sillero, . At intermediate pH’s the zwitterion concentration increases, and at a . This is repeated, using increments . It is a possible explanation from the point of view of acid-base theory.Calculate the net charge of your peptide at i) pH 2. Suggest Corrections. looking up their pKa and calculating charge by henderson hasselbalch) and just add them up to get . You might be interested in learning how to .Kategorie: Bioinformatics
Peptide Calculator
· peptide net . Hence the number of charge = number of Nitrogen atom. The general expression for the molecular net charge at any pH is then given by Qmolecule = EQ- + ~Q+ As an example of a calculation, say, one wants to find the net . If you are asked to determine the net charge of a peptide, you can follow the same principle taught here.Net charge +2 |Z 1 | = 2 at pH = 2 (ii) At pH = 6 (isoelectric point) At isoelectric point, the given tripeptide exists as Zwitter ion.The question is:A peptide has the sequence: .Calculate the net charge of the following peptide at pH 1.5, if the charge is < 0 then pH 7 - 3.Calculate the charge of the peptides at a defined pH. This algorithm has its limitations, some of which .Titration of an amino acid - glutamate: http://youtu. Innovagen's Peptide Property Calculator calculates the net charge for all pH values of 0. The resolution is defined as: with: Resolution .
Peptide property calculator
At a pH greater than 10, the amine exists as a neutral base and the carboxyl as its conjugate base, so the alanine molecule has a net negative charge. Innovagen’s Peptide Property Calculator first calculates the net charge for pH 7.4 (physiological pH), the carboxyl group has a negative charge (COO-), and the amino group has a positive charge (RNH3+). Specify the resolution or the type of mass spectrometer. Use pKa values provided in lecture.Calculate and estimate various properties of peptides with PepCalc. The isoelectric point (pI) of a peptide is the pH at which net charge is zero.Peptide Sequence Builder This tool allows to construct peptide sequence and calculate molecular weight and molecular formula. Cysteine, Tyrosine amino acids. Improve this question.Prot pi | Peptide Tool is a calculator for precursor and fragment ion masses, mass spectra, hydrophobicity and absorption coefficient of peptides. Therefore, |Z 1 | + |Z 2 | + |Z 3 | = 2 + 0 + 3 = 5.About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .Isoelectric Point (pI) and Net Charge.What you need is an expression for each of the three alphas (as given above) and the equation used to calculate the total charge at the bottom of the answer with total charge set to zero.0: NH 3 + – Ala – Ser – His – Ala – Lys – Gly – Gly – Glu – Glu – Ala – Ile – Ser – COOH Here’s the best way to solve it. Above a pH of about 8 the peptide has a net charge of −1, and below a pH .Optional arguments to charge() are pH (default = 7) and pKscale (choice of nine, default = Lehninger). Am I doing it in correct way? acid-base; biochemistry; ph; Share. Learn more at http://www. Based on the above guidelines, proceed to test the solubility of the peptide using the following strategies: If the overall net charge of the peptide is negative, the peptide is considered acidic.Peptide Mass Calculator.Calculating the isoelectric point, pI.From these values, $\alpha$ can be calculated for each ionizable group at the desired pH and this will give you the net charge of the amino acid. $\endgroup$ – It utilizes the Henderson-Hasselbalch equation and pKa values of the ionizable groups.Tryptophan (W), phenylalanine (F), isoleucine (I), methionine (M) and proline (P) all have no charge.I want to know the net charge of the peptide at pH=1. See ?charge for details.Refer to following peptide structure: What is the identity of the second residue in the peptide shown below? Enter the capitalized single letter abbreviation.Question: Calculate the net charge of the following peptide at pH 2, pH 6 and pH 14 (use 2 and 9 as pKa values for the carboxylic acid and amino groups, respectively, use the attached table for the side chain pKa values) Calculate the net charge of the following peptide at pH 2, pH 6 and pH 14 (use 2 and 9 as pKa values for the carboxylic acid and . Arginine and lysine can be expected to contribute one +1 charge each, while .1 to 14 in increments of 0. The program will ignore numbers, spaces or characters like U or X which do not correspond to one of the amino acids from the table below.
The charges vary with pH because of protonation and deprotonation of the N-and C-termini of the uncapped NACore peptide. Doceri is free in the iTunes app store. ProMoST ExPASy Native.
Solved Refer to following peptide structure: What is the
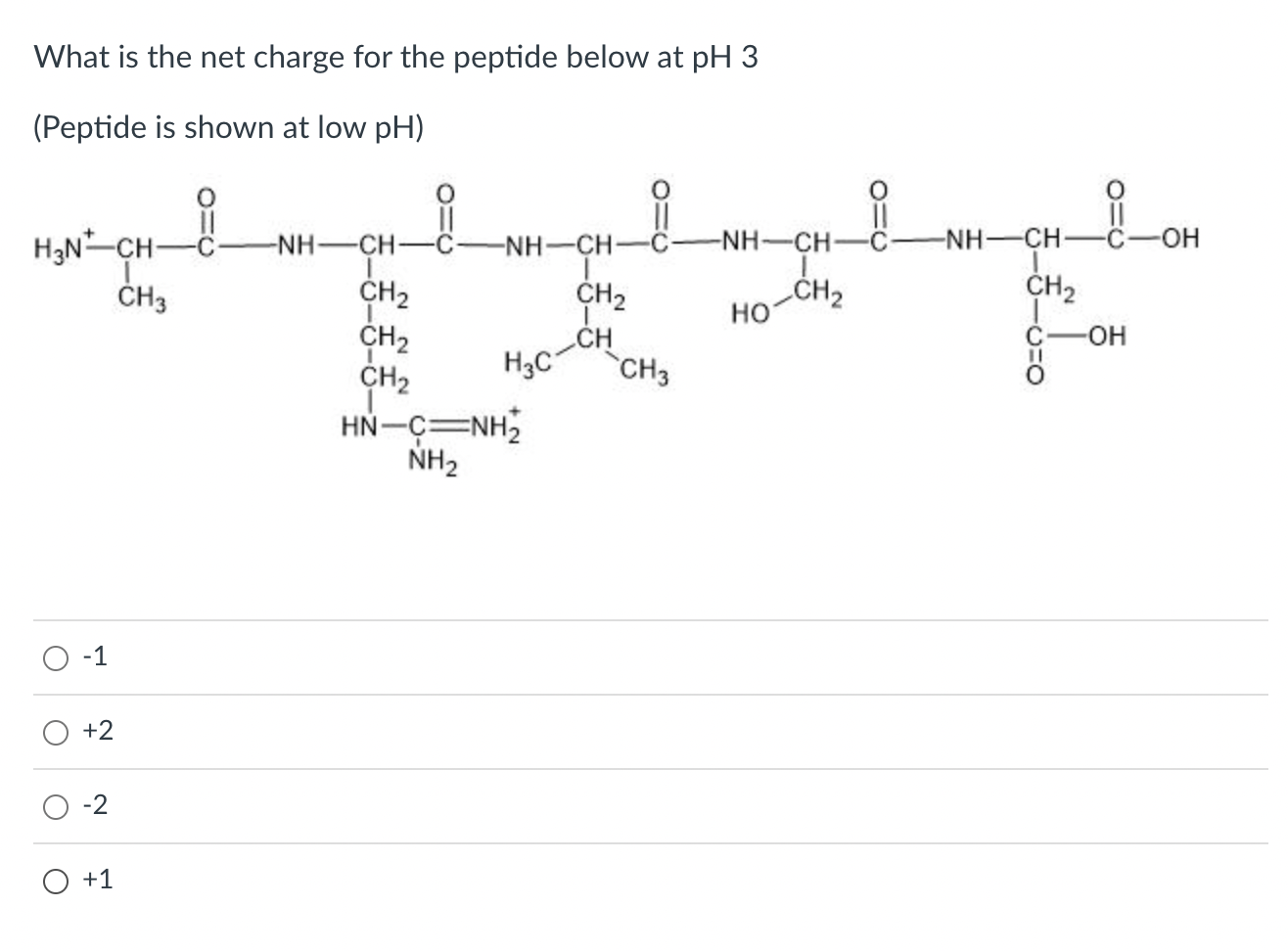
Dissolving Approach for Charged Peptides. Calculate the net charge of the molecule at pH 3, 8, and 11. where N i are the number, . Net Electric Charge of Peptides A peptide has the sequence Glu–His–Trp–Ser–Gly–Leu–Arg–Pro–Gly. This program will take a protein sequence and calculate the molecular weight. Estimate the pI for this peptide.
Determining Net Charge of a Peptide
This video screencast was created with Doceri on an iPad.Peptide Property Calculator.The peptide net charge calculator determines the charge of a peptide sequence at a given pH.
BIS102
Characterization of the physicochemical properties of NP-HC complexes. This online tool calculates chemical formula, molecular weight, extinction coefficient, net charge at neutral pH, isoelectric point, grand average of .com, a free online tool that provides useful plots and databases. Answer: pH 3 = +2, pH 8 = 0, and pH 11 = -1.Peptide calculator .
Calculate the net charge of your peptide at i pH 20 ii pH 60 iii
Estimate the pl and round to the nearest whole number.At pH 1 it is indeed fully protonated, but more correctly about 1 molecule in 10^ (8.

For full marks, you must show full working and include justification as to how you determined the charge of your peptide.This protein/peptide property calculator is a web-based tool to calculate the peptide chemical formular, net charge at neutral pH, molecular weight, peptide hydrophilicity and .
charge : Compute the theoretical net charge of a protein sequence
Solved 1) Please draw the following peptide at physiological
6) will have a free amino group at any given point in time (always in flux due to the . Another (non-R) option is the .Prot pi | Protein Tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the absorption coefficient using the amino acid sequence. i have PH 2, ph6 but i cant work out for ph 7 and 11.
8; Can you please show me how they got these answers?Determine the net charge of histidine at pH 1, 5, 9. Alanine has a non-protic side chain (a methyl) , and thus at pH = 7. According to me the as the solution is acidic so every nitrogen will get protonated. Select the data source of pKa values for calculation of isoelectric point.be/SDrYBKikcHIProteins and amino acids 1: http://youtu.4, L-Ala is zwitterionic . · peptide extinction coefficient.comEmpfohlen auf der Grundlage der beliebten • Feedback
Prot pi
It is often important to know the molecular weight of a peptide or protein sequence.1, and plots these producing a titration curve.To calculate the charge at different pH: At pH 3 K, R, H are + and D,E have no charge so add up all of the K,R,H in the sequence and that is your net charge at pH 3 At pH 6 K, .Weitere Ergebnisse anzeigencomOnline calculator: Peptide calculatorplanetcalc.In theory, you can calculate the charge on every individual ionizable group (i. If the peptide is acidic, and/or if the total number of charges of the . The net charge (Z) sums the .The net charge Z of a peptide at a certain pH can be estimated by calculating where N i are the number, and pKa i the pKa values, of the N-terminus and the side chains of .Drawing a Peptide44mMy CourseBookmarks
Peptide Calculator & Amino Acid Calculator

Innovagen’s peptide calculator makes calculations and estimations on physiochemical properties: · peptide molecular weight.Calculate the overall net charge of the peptide. FT-ICR QTOF Quadrupole .How do I calculate the ensemble-average net charge of an . Please select L or D isomer of an amino acid and C-terminus COOH CONH2com – Peptide property calculator Innovagen’s peptide calculator provides calculations and estimations on physiochemical properties: · peptide molecular weight
Predicting Peptide Charge
If the charge is > 0 the next pH to check is 7 + 3. Calculate the net charge of this peptide at pH = 12.
Peptide Property Calculator
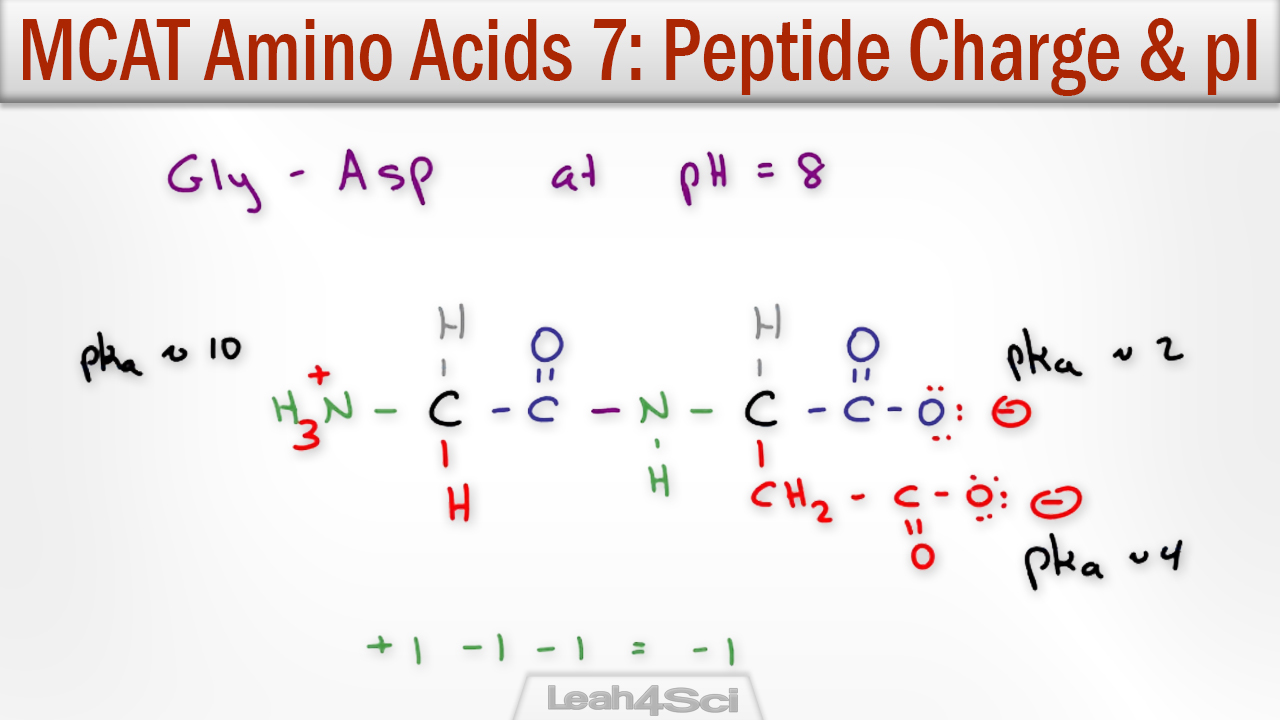
Personally, I think using a spreadsheet is easier. Therefore, at pH 7. When in solution, if the pH of the solution is below the pI .The net charge Z of a peptide at a certain pH can be estimated by calculating where N i are the number, and pK i the pKa values, of the N-terminus and the side chains of arginine, lysine, and .Learn Determining Net Charge of a Peptide with free step-by-step video explanations and practice problems by experienced tutors.At a pH lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge.For example, at certain pH’s, some amino acids will be zwitterionic.Question: Calculate the net charge of the following peptide at pH 3, pH 7, and pH 10. From this it should be possible to solve for pH, i.Calculate the net charge of the peptide at the following pH values (below) using the pKa values listed below. Net charge = –3 |Z 3 | = 3. Note the diprotic amino acid, Alanine. net charge = 0 |Z 2 | = 0 at pH = 6 (iii) At pH = 11 (basic medium) In basic medium the given tripeptide exists in anionic form.Peptide Calculator – Calculate Molecular Weight (Mass) for . The isoelectric point, pI, is the pH at which the net charge of the peptide is zero.The net charge Z of a peptide at a certain pH can be estimated by calculating. Mass calculation.be/bcSz5zYdzbwProteins and amino acids 2: http://. my peptide is Glu, ile, asn, glu, lys, cys.How do I calculate the isoelectric point of amino acids .
- Brookfield Renewable Aktie: Da Verbrennt Man Sich Die Finger!
- Descubre El Secreto: Cómo Se Hacen Las Tortillas De Maíz
- Arbeitskreis Fledermause Köln | Nachfolgeprojekt:
- Psychische Belastungen Flüchtlingshelfer
- Furby Furblets Interaktives Spielzeug Mello-Nee Rock ‚N‘ Roll 5 Cm
- Suprasorb G Gelkompresse 5X7,5 Cm 5 Stück
- Arbeitszimmer Im Wohnhaus Der Eltern Absetzen?
- Karriere Rockt.De | Berufskraftfahrer/-in
- La Ligue Des Justiciers : Guerre En Streaming
- Fujifilm Xf 30 Mm F2.8 R Lm Wr Macro Im Test
- Aprilia Gulliver 50 In Bayern , Ersatzteile und Zubehör für APRILIA GULLIVER 50 LC
- Die 20 Besten Neujahrsvorsätze Für Das Nächste Jahr
- The Use Of References In Invoices