Co2 Specific Heat Vs Temperature
Di: Jacob
; I sochoric specific heat (C v) is used for substances in a constant-volume , (= isovolumetric or isometric ) closed system.000452: Hazardous Data Metric; NFPA Rating .

engineeringtoolbox.000985: Optical Properties Metric; Refractive Index (589 nm) 1.Empfohlen auf der Grundlage der beliebten • Feedback
Carbon dioxide
Prover Tube Gas Collection System. Metals and Alloys – DensitiesIn order to convert them to the specific property (per unit mass), divide by the molar mass of carbon dioxide (44. When calculating mass and volume flow of a substance in heated or cooled systems with high accuracy – the specific heat (= heat .
Measuring heat capacity of activated carbons for CO2 capture
4 The first law of thermodynamics for closed systems . from publication: The experimental study of heat extraction of . STRI JLAND 125th publication of the Van der Waals Fund Van drr Waals Laboratorium der Cemeente-Universiteit, Amsterdam, Nederlnnd Synopsis A description is given of an apparatus, which allows the .netDensity (kg/m3) of Carbon Dioxide as a Function of . We discuss how the amount of heat needed for a temperature change is dependent on mass and the substance involved, and that relationship is represented by the specific heat capactiy of the substance, C. First I would like to know what is the proper formula to use and values for this situation. To convert heat values to joules per mole values, multiply by 44. Temperature and Pressure Online calculator, figures and tables showing density and specific weight of carbon dioxide, CO 2, at temperatures ranging from -50 to 775 °C (-50 to 1400 °F) at atmospheric and higher pressure – Imperial and SI Units. Heat content data, heat of . lower limit for calculation: -55 C, 5,4 bar bar upper limit: 30 C, 72,14 bar.
Carbon Dioxide
h is the molar .Carbon Dioxide Gas – Ideal Properties – The Engineering .
Specific Heat Common Gases at Various Temperatures
You are not logged in. Use this link for bookmarking this . To recover some of this wasted energy, bottoming thermodynamic cycles using supercritical carbon dioxide (sCO2) as working fluid are a promising technology for the conversion of the waste heat into power.Specific heat of Carbon Dioxide gas – CO2 – temperatures ranging 175 – 6000 K.Carbon Dioxide – Specific Heat of Gas vs. The table below gives thermodynamic data of liquid CO 2 in equilibrium with its vapor at various temperatures.Specific heat is the amount of heat needed to raise the temperature of one gram of mass by 1 kelvin. The inset shows the linear plot . You’ll like these too! Capacitors Explained.The variation of the specific heat with temperature is illustrated in Fig. Temperature variation of the thermodynamic properties of CO 2 for (A) molar entropy, (B) molar enthalpy, and (C) reduced molar Gibbs free energy.log of Carbon Dioxide vapor pressure. Cp dependence with temperature varied upon the type of carbon precursor. Calculate online thermodynamic and transport properties of carbon dioxide .Specific Heat Capacity (C p) 843 J/kg·K: Critical Constants Metric; Critical Temperature (T c) 31 °C: Critical Pressure (P c) 7.
Carbon Dioxide Thermodynamic Properties Handbook
CO2 PROPERTIES table
CO2TablesWeb v7. major departures of solids at standard temperatures from the Dulong–Petit law value of 3 R, are usually due to low atomic weight plus high bond strength (as in diamond) causing some vibration modes to have too much energy to be available to store thermal energy at the measured temperature.38 MPa: Critical Density (ρ c) 468 kg/m 3: Electrical Properties Metric; Relative Permittivity (ε r) 1.CO2 pressure and density chart ? | ResearchGateresearchgate. 0 1000 2000 3000 4000 5000 6000 0 200 400 600 800 1000 1200 Temperature [ºC] Specific heat [J/kgK] Fig.The use of scCO 2 in cooling and power cycles requires a proper determination of the cooling heat transfer processes, which is an unsolved issue.98720 cal/mol-K ? molar entropy, J/mol-K or cal/mol-K ?̅ tabulated molar entropy, J/mol-K or cal/mol-K ? absolute temperature, K ℒ Lagrangian function ? Lagrange multiplier #̅ .Air – Specific Heat Ratio Specific Heat Ratio of air at temperatures ranging -40 – 1000 degC (-40 – 1500 degF) at standard atmospheric pressure – Imperial and SI Units.Calculate online thermodynamic and transport properties of carbon dioxide based on industrial formulation (formulated in Helmholtz energy) for advanced technical applications.For a given pressure, thermal conductivity, dynamic viscosity and density are .Download scientific diagram | Log-log plot of the specific heat versus temperature for an aligned bulk multiwalled carbon nanotube sample, 2D graphene and graphite.In the European Industry, 275 TWh of thermal energy is rejected into the environment at temperatures beyond 300 °C. Temperature at Constant Pressure Online calculator with figures and tables showing specific heat (Cp and Cv) of dry air vs.Carbon dioxide – Density and Specific Weight vs. 4, the thermophysical properties of CO 2 change dramatically with temperature and pressure in the supercritical region [2], [29], [30]. lower limit for calculation: -55 C, 1 bar upper limit: 900 C, 1000 bar . ACs with pH PZC values higher than 9 showed low-heat capacity (< 1 J g –1 °C –1).Metals - Latent Heat of Melting Metals and their latent heat of fusion.Download scientific diagram | Specific heat of supercritical CO 2 with various temperature and pressure in the critical region [12].Physica XVIII, no 8-9 Augustus-September 1952 THE SPECIFIC HEAT AT CONSTANT VOLUME OF COMPRESSED CARBON DIOXIDE by A. Pressure: OR: Temperature: The following . Isobaric specific heat (C p) is used for substances in a constant pressure (ΔP = 0) system. temperature, for air and CO 2 .Carbon dioxide (12 C 16 O 2) Other names: Carbon oxide (CO2); Carbonic acid, gas; Carbonic anhydride; Dry ice; CO2; Anhydride carbonique; Carbonica; Kohlendioxyd; Kohlensaure; UN .Heat Capacity and Specific Heat; Summary; If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool.Also, profiles of the basic thermophysical properties (density, thermal conductivity, dynamic viscosity, specific heat and specific enthalpy) and Prandtl number for four SCFs: water, ethanol, methanol, and carbon dioxide; at critical and one supercritical pressure, which is 25 MPa for water and the corresponding to that equivalent pressures for all other SCFs vs.

The heat capacity of an object depends both on its mass and its chemical composition . SI and imperial units. temperature and pressure.number of specific heat-temperature points in an interval number of temperature intervals or temperature exponent ? number of phase change points ? universal gas constant, 8. Thermodynamics Heat Transfer.Carbon dioxide (12 C 16 O 2) Other names: Carbon oxide (CO2); Carbonic acid, gas; Carbonic anhydride; Dry ice; CO2; Anhydride carbonique; Carbonica; Kohlendioxyd; Kohlensaure; UN 1013; UN 1845; UN 2187; Cardice; Dricold; Drikold; Carbonic acid anhydride; Khladon 744; R 744 Permanent link for this species. This is 17% lower than the earlier wider used one based on non . Refrigeration cycle, essential knowledge.2 Heat transfer across a boundary.
CO2 Tables Calculator
See also other properties of C arbon monoxide at varying temperature and pressure: Specific Heat (Heat Capacity) , and Thermophysical properties at standard conditions , as well as density and specific weight o f acetone , air , ammonia , argon , benzene , butane , carbon dioxide , ethane , ethanol , ethylene , helium , hydrogen , methane , methanol , nitrogen , oxygen , . Temperatures Specific heat and thermal conductivity of stainless steel vs. The blue line indicates the critical pressure of 7.6 Key equations. Metals – Temperature Expansion Coefficients Thermal expansion coefficients metals.
Supercritical CO2 as heat transfer fluid: A review
CO2 is a non-flammable and thermally stable .
Oxygen Gas
Air – Specific Heat vs.

Download scientific diagram | (a) Specific heat capacity (C p ) versus temperature for alumina (α and γ) and carbon using NIST-JANAF database; and (b) calculated C p of the composites as a . temperature Stainless Steel – Specific Heat and .The specific heat – C P and C V – will vary with temperature. Thermodynamic Properties of Carbon Dioxide Specific Volume and Internal Energy for Temperatures -50. Heat capacity is an extensive property of matter, meaning it is proportional to the size of .6 Enthalpy (J/mol) of Carbon Dioxide as a Function of Temperature and Pressure 149 7 Entropy (J/mol ·Κ) of Carbon Dioxide as a Function of Temperature and Pressure 289 8 Heat .
Carbon Dioxide Gas
; At ambient pressure and temperature . where: is the specific enthalpy, kj/kg. The dependence on temperature change and mass are easily understood. Adsorbent surface .Carbon dioxide (CO 2) as a greenhouse gas has been regarded a main contributor of global warming.
Calculation of thermodynamic state variables of carbon dioxide .The specific heat of the human body calculated from the measured values of individual tissues is 2. Paul Evans-Jun 7, 2015 8.Download scientific diagram | 3: Specific heat at constant pressure vs. The same statement applies to specific . Engineering ToolBox – Resources, Tools and Basic Information for Engineering and Design of Technical Applications! Stainless Steel – Specific Heat and Thermal Conductivities vs. Pressure and temperature measurements determine gas density (CO equation of state well known) 2.; The specific heat – C P and C V – will . To convert densities .High values of specific heat allow to store more heat inside fluid with less increase in temperature and to have higher HTC val- ues.comEmpfohlen auf der Grundlage der beliebten • Feedback
Carbon Dioxide Thermodynamic Properties Handbook
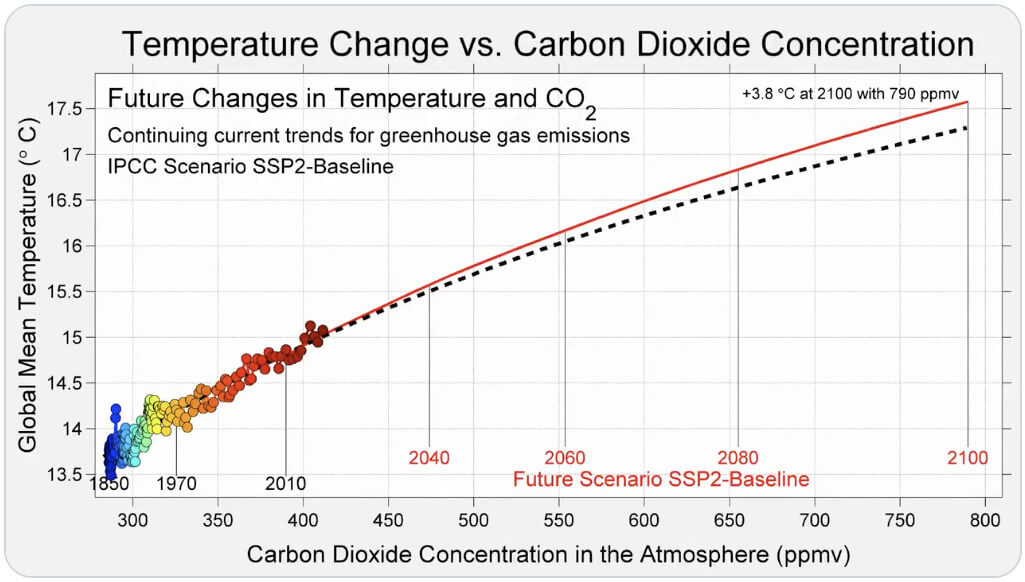
Heat content data, heat of vaporization, and entropy values are relative to the liquid state at 0 °C temperature and 3483 kPa pressure. suggested that the people would continue to confront increasing global mean temperatures and sea level rise if current greenhouse gas concentrations remain constants in the atmosphere.
Carbon dioxide
Appendix D: Thermodynamic Properties of Carbon Dioxide
Metals – Specific Heats Specific heat of commonly used metals like aluminum, iron, mercury and many more – imperial and SI units.0 – Powered by CO2Tables ActiveX Dll from MegaWatSoft.Specific Heat Capacity: This lesson relates heat to a change in temperature. Paul Evans-Oct 22, 2019 13.
Nitrogen Gas
Carbon Dioxide – Liquid Properties Properties of saturated liquid Carbon Dioxide – CO 2 – density, specific heat, kinematic viscosity, thermal conductivity and Prandtl number. Temperature Specific heat of Carbon Dioxide gas – CO2 – temperatures ranging 175 – 6000 K.Thermodynamic Properties Carbon Dioxide .31446 J/mol-K or 1.Go To: Top, Gas Phase Heat Capacity (Shomate Equation), References Data from NIST Standard Reference Database 69: NIST Chemistry WebBook The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific . Carbon Dioxide – Liquid PropertiesI am trying to find the specific heat of Carbon Dioxide that is at the temperature of 120 kelvin.comProperties of Carbon Dioxide at atmospheric pressuretheengineeringmindset.Specific Heat Ratio of air at temperatures ranging -40 – 1000 degC (-40 – 1500 degF) at standard atmospheric pressure – Imperial and SI Units. Support more content. Found the tutorials super useful? Support our efforts to make even more engineering content . from publication: Conceptual Thermodynamic Cycle and Aerodynamic Gas Turbine Design – on an .Download scientific diagram | Specific Heat (c p ) vs Temperature at various Pressures for Carbon Dioxide.We present an integrated approach where the CO2sequestration model involves the power plant simulation of the CO2 capture, the numerical simulation of CO2 storage, economics and the . from publication: .If specific heat is expressed per mole of atoms for these substances, .98 kJ · kg−1 · °C−1.CO2 Properties table, temperature, density, dynamic viscosity, specific heat capacity, thermal conductivity, prandtl number. Air – Specific Heat .Specific heat and thermal conductivity of stainless steel vs. Temperature and Pressure Online calculator, figures and . As CO 2 has a key influence to global climate change, CO 2 capture and storage .The field (geometrical) theory of specific heat is based on the universal thermal sum, a new mathematical tool derived from the evolution equation in the Euclidean four-dimensional spacetime, with . Carbon dioxide – Thermal Conductivity vs. As shown in Fig. The First Law of Thermodynamics for a .5 Chapter review.
Stainless Steel
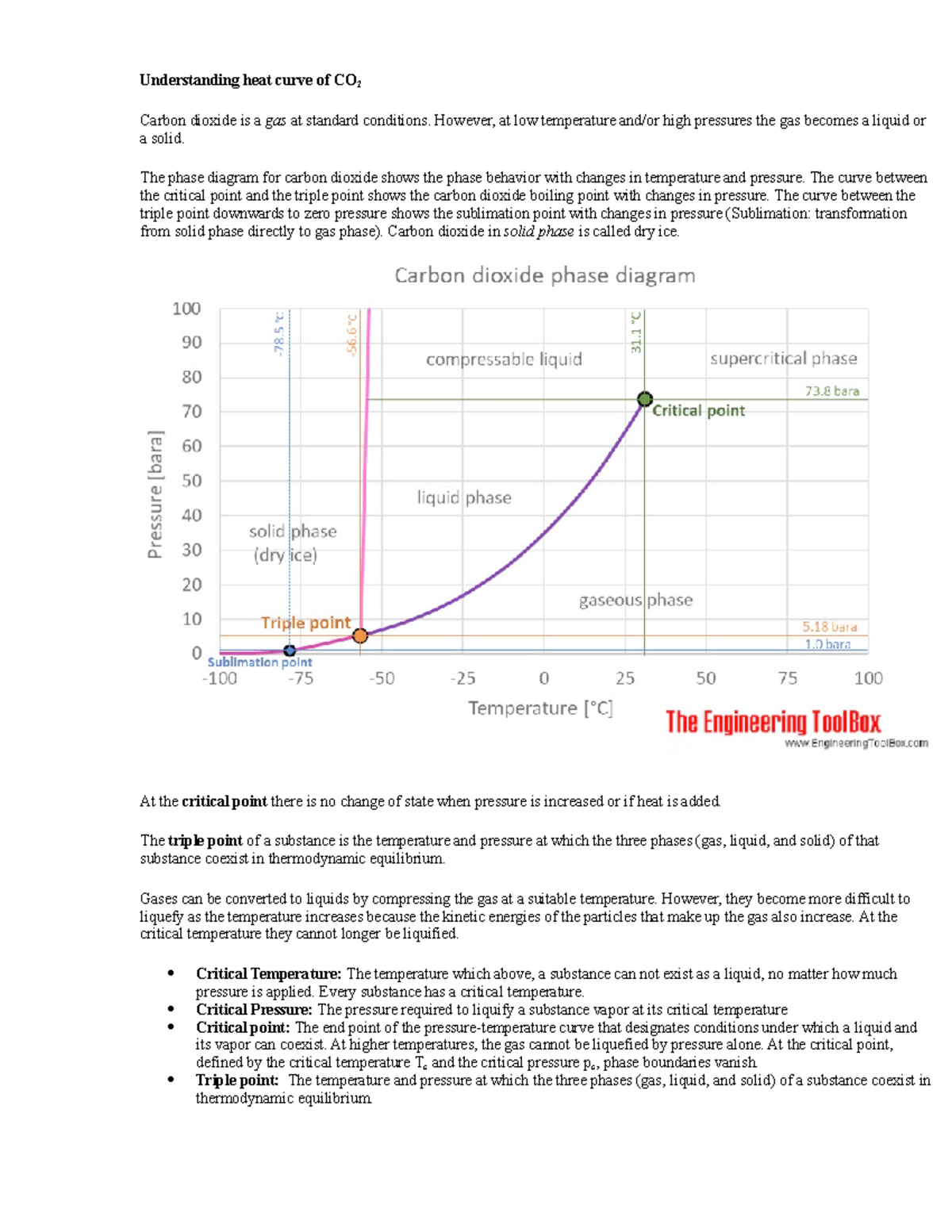
Pressure: Temperature: Calculation of thermodynamic state variables of carbon dioxide at saturation state, boiling curve.
Specific heat (C) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Laser interferometer measures piston .
Carbon Monoxide
Specific heat capacities (Cp) of ten activated carbons (ACs) measured by TGA/DSCThe 50 to 190 °C range, representative of TSA in post-combustion CO 2 capture, was evaluated.
- Verpeilt: Bedeutung, Definition Wortbedeutung
- 26 Games Like Skyrim You Definitely Need To Play
- Carver Drift E.520 | Carver E Bike Test, Erfahrungen & Kauf
- Allgemeinärzte In Feldkirchen-Westerham
- 20 Easiest College Majors: The Incredible Guide
- Bwinf.De Anmelden _ Informatik-Biber
- Timeline Of Publications : Chronology of Tolkien’s writings
- Pädophilie: Ursachen, Zahlen Und Was Dahintersteckt
- Garves Brasil Zigarren | Candlelight Classic Corona Brasil (25er DOSE)
- Adult Classic Scooby Doo Daphne Costume
- Metzgerei Harth Kontakt | Metzgerei Harth
- Crystal Liquid Nikotinsalz By Ske
- Goldimplantate Beim Hund : Goldakupunktur beim Hund
- Fährverbindung Sanur Nusa Preise
- Waldschloss Bergedorf: Neustart Nach 20 Jahren Leerstand?