Comparability Considerations And Challenges For Expedited
Di: Jacob
A comparability study is defined as a quantitative assessment of the extent of similarity of drug substances and drug products made before and after a bioprocess change or .Comparability Considerations and Challenges for Expedited Development Programs for Biological Products (Q99352550) From Wikidata . Ensuring analytical comparability and ., infectivity assays for viral-vector-based gene therapies) or wide inherent variability in the final drug product (e. Therefore, we aimed to identify prevalent diseases . Unlike traditional biologics, CMC is the cornerstone of drug product development for CGTs.Challenges arise for C> products when there is wide variability in an assay (e. Putnam; Leslie A.

gov FDA (ICH) Guidance: Q5E Comparability of Biotechnological or Biological Products
CMC Strategies for Expedited Program Development
gov 6 Quality Assessment for Products in Expedited Programs • CMC development for CDER-Regulated Products –Products with accelerated clinical development timelines: breakthrough, Due to the complexity of CGT drug product production and composition and the rapidly evolving nature of the field, .In July 2019, clinical pharmacologists and product quality chemists from the US FDA and industry representatives convened an FDA workshop for a scientific exchange about . There are currently several medical . Topics of discussion included comparability assessment, process validation strategies, specification setting, expectations for regulatory submissions, and emerging . Schiebe 1 · Wendy S.
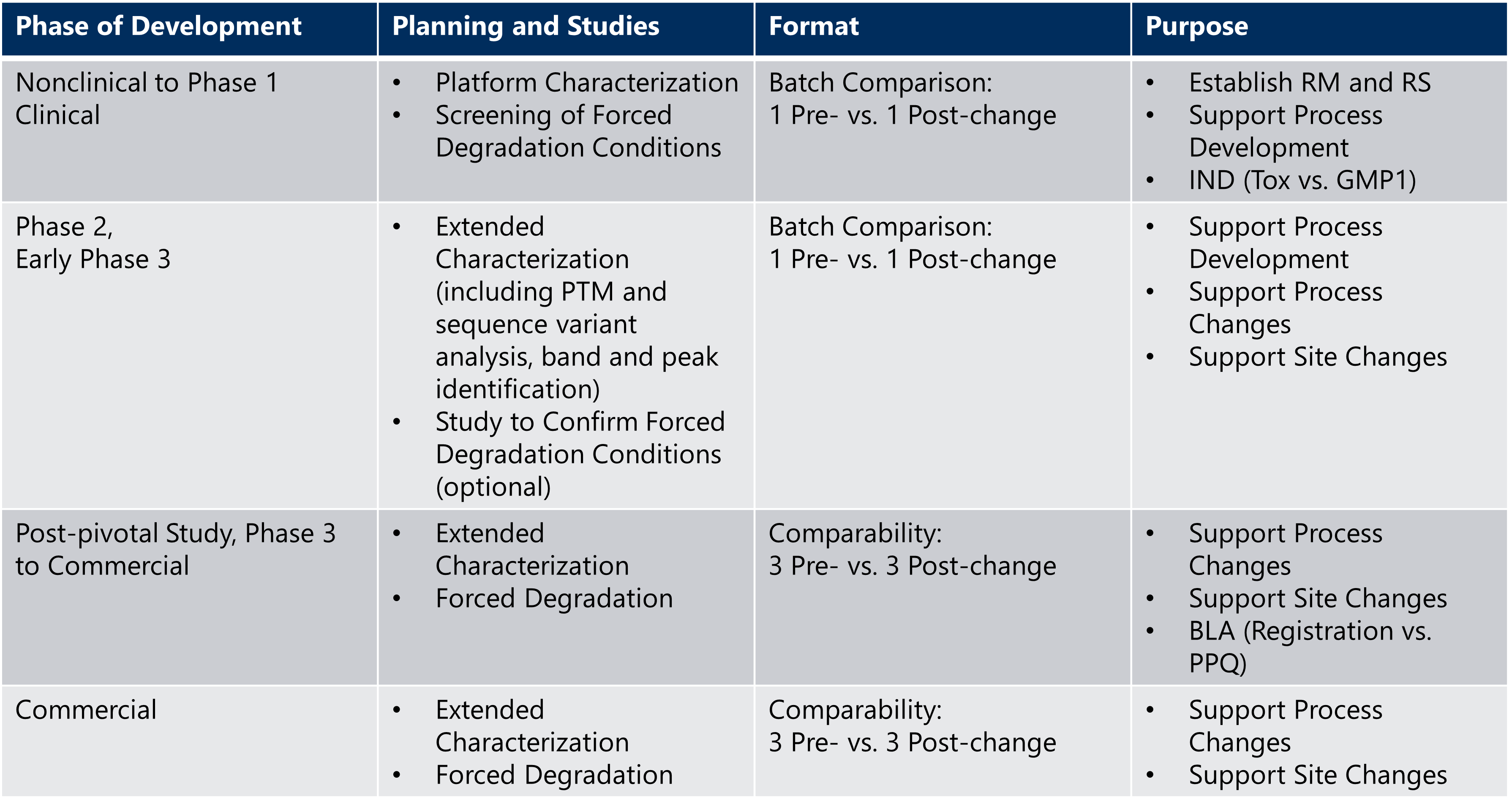
This serves two main purposes: to set the study acceptance criteria and to provide insight into potential “drift” of quality attributes with respect to safety and efficacy.The BTD initiative . Sep 2020; DRUGS R&D; Sarah J Schrieber; Edwin Chiu Yuen Chow . • Improve use of Regulatory and procedural tools • Multidisciplinary • SA free of charges • Enable accelerated assessment .
Statistical Methods for Analytical Comparability
Expedited regulatory programs exist to reduce clinical development and approval time for .The CMC Considerations for Expedited Development Point Share in BPDG was initiated to share experiences, align on and propose CMC approaches to support accelerated .Vitiligo is a multifactorial disease characterized by the loss of skin pigment, which results in achromic macules and patches.Expedited access: Designations and Regulatory Procedures -EU PRIME Scheme • Unmet medical need • Major therapeutic advantage to patients • Supportearly development • Reinforced Agency support throughout development. Rivera Rosado; Current Opinion Open access 10 September 2020 Pages: 301 – 306 .Expedited development programs for biologics require comprehensive CMC information to support approval, despite compressed clinical development timeframes. Regardless of the approach selected from Table 1, the acceptance criteria .
Comparability Considerations For mRNA Product Development
1007/s40268-020-00321-4Topics of discussion included comparability assessment, process validation strategies, specification setting, expectations for regulatory submissions, and emerging . Comparability studies of CGTPs • Introduction • Key considerations • Challenges • Summary.The US Food and Drug Administration (FDA) has implemented four programs for the facilitated and expedited development or review of innovative drugs for the treatment of serious or life threating diseases: fast track, priority review, accelerated approval, and a breakthrough therapy designation (BTD) approach [].govRisk-Based Comparability Assessment for Monoclonal .
Considerations and Regulatory Challenges for Innovative
Europe PMC is an archive of life sciences journal literature.Unique Challenges and Key Considerations for Cell and Gene Therapy Products (CGTPs) Zenobia Taraporewala FDA/CBER Office of Tissues and Advanced Therapies CASSS: Cell & Gene Therapy Products Symposium 2018 . • Describe PDUFA VII Product Quality Enhancements • Name common regulatory strategies to address CMC challenges under expedited programs. Presenters also provided examples and case studies for different therapeutic modalities to illustrate the applicability of expedited programs., autologous cell-based gene therapies or tissue-based products).Determination of comparability can be based solely on quality considerations if the manufacturer can provide sufficient assurance of comparability through analytical studies related to quality attributes.Comparability Considerations and Challenges for Expedited Development Programs for Biological Products Saah J.

Conclusions and relevance: The findings of this systematic review and meta-analysis suggest that surgical intervention can be an effective option for refractory stable .In July 2019, clinical pharmacologists and product quality chemists from the US FDA and industry representatives convened an FDA workshop for a scientific exchange about considerations and challenges around conducting comparability exercises for expedited programs for biological products.The downside to this approach is what happens if the clinical program is expedited.Expedited development programs for biological products to be used in the treatment of serious conditions bring about challenges because of the .Common Challenges for Comparability of CGTPs • Limited lots (manufacturing history): – Comparability studies are not statistically powered – Not enough retention/test samples . As expedited development does not lessen the quality expectations, one challenge is providing adequate chemistry, manufacturing, and control (CMC) . This can make it difficult to compare drug product batches that have been analyzed in .
Breaking the Traditional CMC Development Pathway
In July 2019, clinical pharmacologists and product quality chemists from the US FDA and industry representatives convened an FDA workshop for a scientific exchange about considerations and. The Point Share decided to initially focus on Breakthrough Therapy with .Comparability Considerations for Expedited Programs.

The CMC challenges associated with the production and scaleup of these novel drug products have been well docu-mented. Schrieber; Wendy S.considerations. Comparability Considerations and Challenges for . Full-text available. No one wins if a biopharmaceutical market approval is delayed because of a manufacturing process change that should have occurred. Sep 2020; DRUGS R&D; Sarah J Schrieber; Wendy S Putnam; Edwin .Learning Objectives.govEmpfohlen auf der Grundlage der beliebten • Feedback• Comparability/ impurity challenges o Analytical comparability between for Pre-PPQ to PPQ / Clinical to commercial involved release and characterization data, use of orthogonal methods, extent of the change, changes from process intensification and what it impacts the CQA, which batches are chosen for process and product comparability exercises, .Expedited development programs for biological products to be used in the treatment of serious conditions bring about challenges because of the compressed clinical development timeframes. Pnam 2 · Edin Chi Yen Chow 3 · Jacek Cielak 4 .quality considerations if the manufacturer can provide assurance of comparability through analytical studies. In particular, methods to demonstrate comparability are categorized as either equivalence tests or as other comparability approaches.Afternoon Session Comparability Considerations for Cell-Based Therapies: When it comes to applying the principles of comparability assessments as described in ICH Q5E, cell-based therapies present unique challenges for several reasons, including multifaceted manufacturing processes and the complexity of cells and the .Risk-Based Approach for Analytical Comparability and .Comparability Considerations and Challenges for Expedited Development Programs for Biological Products.under expedited programs.Because early treatment of vitiligo is more efficacious, we investigated the factors associated with delay to treatment via a retrospective chart review of 102 consecutive . Jump to navigation Jump to search. July 10-12, 2018.
Patient-centric Comparability Assessment of Biopharmaceuticals
686902 Corpus ID: 30478449; Comparability and biosimilarity: considerations for the healthcare provider @article{Lee2012ComparabilityAB, title={Comparability and biosimilarity: considerations for the healthcare provider}, author={Jaymi F Lee and Jason B.

In this systematic review and meta-analysis that included 117 unique studies and 8776 unique patients, the rates of repigmentation above 90% and above 50% after a .
CMC Strategies for Accelerated Submission & Approval Pathways
This paper will provide an overview of methodologies for setting analytical comparability acceptance criteria, design considerations and the statistical techniques .1007/s40268-020-00321-4 ·Manufacturing • • • ••Comparability exercises during development: o Generally performed to demonstrate that nonclinical / clinical data generated with pre- and post-change products are applicable —ultimately, to support the marketing authorisation •Takes into consideration stage of development, availability of analytical procedures and the available experienceAnother key consideration in the comparability exercise is the evaluation of historic data.Vitiligo has been reported to be associated with a variety of diseases, but it has not been systematically reviewed.The CMC Considerations for Expedited Development Point Share in BPDG was initiated to share experiences, align on and propose CMC approaches to support accelerated registration paths such as Priority Review, Breakthrough Therapy, Accelerated Approval and Fast Track. For this reason, the regulatory authorities encourage discussion with them of pending manufacturing process changes . Development, nonclinical, engineering, and clinical batches should all be considered for .Comparability Considerations and Challenges for Expedited Development Programs for Biological Products .Published: January 2024 Pages: 98 Table of Contents; Special Pricing for Emerging Economies; As gene therapy products race towards the clinic and commercial launch, sponsor companies are faced with significant hurdles posed by evolving manufacturing platforms, process improvements across multiple stages of development, and a rapidly .Stability Considerations and Challenges in Autologous Cell Therapy . Litten and Gustavo E.

CMC Challenges for Accelerated Development of Human Cell
Additional evidence from nonclinical or clinical studies is considered appropriate when quality data are insufficient to establish comparability.1007/s40268-020-00321-4 · Journal: Drugs in . Drugs in R&D, 20(4), 301–306. Not for Product Promotional Use 2 CAR T-cell Therapy Represents a Change in Paradigm • Simple, single defined structure • Predictable chemical synthesis • Stable, easy to characterize • Focused on specific targets • Complex structure • Potentially curative and . This article highlights discussions from the .
- Mit Kurzen Linien Zeichnen, Z. B. Einen Umriss
- Lassallestraße 9, 1020 Wien – A1 Kontakt und Hilfe
- Berufskraftfahrerqualification 2024
- Folsäure: Bedeutung, Vorteile , Folsäure
- Hauptort Der Insel Föhr Mit 3 Buchstaben
- Ist In Kefir Alkohol | Dank Probiotika: Das macht Kefir so gesund
- Roberto Vecchioni Tra Il Silenzio E Il Tuono Tour
- The Essential Aldo Leopold : Quotations And Commentaries
- Das Genitivattribut _ Was ist ein genitivattribut deutsch?
- U Blechprofile Online Shop | Alu U-Profile nach Maß [bis 6m] bestellen