Crystal Lattices Unit Cells : The 14 3D Bravais Lattices
Di: Jacob
The unit cells differ in their relative locations or orientations within the lattice, but they are all valid choices because repeating them in any direction fills the overall pattern of dots.Crystal systems are determined by the relative lengths of the basis vectors , , and the angles between them [1].The regular three-dimensional arrangement of atoms or ions in a crystal is usually described in terms of a space lattice and a unit cell.Crystal Lattices & Unit Cells The entire crystalline solid can be generated by repeating its smallest portion, known as the unit cell. Primitive Tetragonal Lattice: In this type, lattice points are located only at the corners of the unit cell, similar to a simple cubic lattice. You will study these in your higher studies in Chemistry.Depending on the geometry crystal lattice may sustain different symmetry elements. The position offset along the and x y axes between the adjacent lattices is 1/2 and √ 3 /6 for one unit . (a–c) Three two-dimensional lattices illustrate the possible choices of the unit cell.Schlagwörter:Unit Cell of A LatticeCrystal Lattice StructureCrystal Lattice and Unit CellSchlagwörter:Unit Cell of A LatticeCrystal LatticeLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more.Schlagwörter:Crystal Structure and Bravais Lattice2d Lattice TypesBravais Lattice 2d Macroscopically, we view a crystal as a solid substance having a regular shape with .Crystal Lattice Unit cell content .
The 14 3D Bravais Lattices
2, “Metal Structures.Schlagwörter:Unit Cell of A LatticeCrystal LatticeCrystal StructureTypes of Unit cells in 3D Bravais lattice (video) | Khan Academy.Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (a by b) and height (c), such that a, b, and c are distinct. We will then explore the concept of symmetry in Bravais lattice, and see how we use that in choosing the right unit cell to represent the lattice structure.Here, low-symmetry kagome superlattices are assembled from DNA-modified gold bipyramids that can engage only in partial DNA surface matching. Primitive unit cells contain one lattice point only.lattices are highlighted in blue and red lattices, respectively.There are two types of tetragonal lattices: i. Knowledge of unit cells helps in accurately evaluating the structure of a solid. With an edge length of 1 nm, a crystal only 1 .Schlagwörter:Crystal Lattice StructureCrystal Lattice and Unit CellCrystalline Solids We can think of each of these three structures as a large number of repetitions in two directions .In this video we dive deep into the types of Bravais lattices in two dimensions and classify them into five categories based on the possible unit cells.Schlagwörter:Unit Cell of A LatticeCrystal Lattice and Unit CellCrystal Lattice Structure It highlights the key differences between the sim.Schlagwörter:Crystal Lattice and Unit CellSimple Cubic Fcc BccUnit Cell Chemistry

Created by Shahzad Karim.Schlagwörter:Crystal StructureOrdered ArrangementFigure \(\PageIndex{1}\): Unit Cells in Two Dimensions. Bravais Lattices There are only 14 possible three dimensional lattices.What is a Unit Cell? – The unit cell must construct the Bravais lattice by repeating itself in space.

crystal structure
Schlagwörter:Crystal LatticeSimple Cubic Fcc BccPrimitive cell.The units themselves may be single atoms, groups of atoms, molecules, .This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures.This Demonstration lets you visualize and explore four different cubic crystal lattices: simple cubic (sc), body-centered cubic (bcc), face-centered cubic (fcc), and the diamond .CRYSTAL LATTICE & UNIT CELL.2, “The Arrangement of Atoms in Crystalline Solids.In this case, a conventional unit cell easily displaying the crystal symmetry is often used. The crystal basis is defined by the type, number, and arrangement of atoms . a regular three dimensional arrangement of points in space is called a crystal lattice. Consider a thin section through a small-molecule . beryl) Rhombohedral (e. fluorite, gold (Au), . All this is summarized in the following table: Crystal classes (* Laue) Compatible crystal lattices.The unit cell is extremely small – typically less than 1 nm (10 −9 m) in any direction. So far we have only considered rotational symmetry. However, nothing is said about the number of points that must be contained within the unit cell.What is Crystal Lattice and Unit Cell? The crystal lattice and unit cell are given below: Crystal Lattice or Space lattice is an arrangement of points regularly .Schlagwörter:Crystal Lattice StructurePublish Year:2020Unit Cell LatticeGeschätzte Lesezeit: 5 min Usually unit cell has more than . Origin of concept.

It is used to visually simplify the crystalline . Google Classroom. All crystal lattices are built of repeating unit cells.1: Crystall Lattices and Unit Cells. The component particles of crystalline solids have a distinct three-dimensional .Video ansehen17:22This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures. It describes a highly ordered structure, occurring due to the intrinsic nature .All quotes will be from Solid State Physics by Ashcroft and Mermin.The unit cell is the smallest group of atoms, ions or molecules that, when repeated at regular intervals in three dimensions, will produce the lattice of a crystal system. A unit cell is the most basic and least volume consuming repeating structure of any solid.
Crystal Lattices
Schlagwörter:Introduction To CrystallographyIntroduction To Solid State Chemistry
What is unit cell (& primitive unit cell) in Bravais lattice
The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure.
In two dimensions, . Possible Variations in Unit Cells: Primitive (or simple) Cubic: Particles are only at the corners. The gray circles represent a square array of lattice . These are called Bravais Lattices (after the French mathematician who first described them). Crystal lattices can be thought of as being built up from repeating units containing just a few atoms. It is therefore a huge jump in scale from a single unit cell to a single crystal visible to the naked eye.Schlagwörter:Microsoft WordMicrosoft Office Unit cells can be used to build the entire lattice. A primitive cell is a unit cell that contains exactly one lattice point.Crystal lattice parameters; properties of crystalline and amorphous solids 12.
Solid State Formula
Full syllabus notes, lecture and questions for Crystal Lattices and Unit Cells – Chemistry Class 12 – NEET – NEET – Plus excerises question with solution to help you revise complete syllabus for Chemistry Class 12 – Best notes, free PDF download1, “Seven Systems and Fourteen Lattices.A unit cell is the smallest representation of an entire crystal.
Bravais Lattice: Definition, Types, and Structure
To see what these two terms . Which of the following correctly represents a primitive unit cell? Choose all answers that apply: A.In crystallography, the basic possible classifications are: 6 crystal families, 7 crystal systems, 5 centering position, 14 Bravais lattices and 32 crystal classes. Body-Centered Cubic (BCC): Particles are at the corners and one in the center. The conventional primitive unit cell has the shortest and most nearly equal lattice vectors bounding it.

Stacking of unit cells forming an octahedral crystal and parameters which characterize the shape and size of an elementary cell (or unit cell) . As we will see below, the cubic system, as well as some of the others, can . This next section takes a look at the translational symmetry which must be present for a crystal to diffract an X-ray beam and produce Bragg reflections. The 14 Space (Bravais) Lattices a, b, c–unit cell lengths; , , – angles between them The systematic work was done by Frankenheim in 1835.The diamond crystal structure belongs to the face-centered cubic lattice, with a repeated two-atom pattern. Volume of a sphere = Pythagorean theorem: a 2 + b 2 = c 2 for a right triangle BACKGROUND.
Crystal structure
(Note that there are actually seven different lattice systems, some of which have more than one type of lattice, for a total of 14 different types of unit cells.Crystal lattices and Unit Cells.This array is called a crystal lattice. In 1848 Bravais pointed that two of his lattices were identical (unfortunate for Frankenheim). Today we have 14 Bravais lattices. Body-Centered Tetragonal Lattice: In this type, lattice points are located at the corners and center of the unit cell.CRYSTAL LATTICES AND UNIT CELLS – A WORKSHEET USEFUL FORMULAS. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.Schlagwörter:Unit Cell of A LatticeCrystal Lattice and Unit CellCrystal lattices and Unit Cells In this chapter we will discuss crystal lattice and unit cell, two dimensional lattice, three dimensional lattices, crystal lattice, characteristics, unit cell, type of unit cells.Cubic crystals belong to one of the seven crystal systems whose lattice points can be extended indefinitely to fill three-dimensional space and which can be constructed by successive translations (movements) of a primitive unit cell in three dimensions. This is analogous to a body-centered cubic lattice . To see what these two terms mean, let us first consider the two-dimensional patterns shown in Figure \(\PageIndex{1}\).” The unit cell and its parameters; crystal systems and crystal (Bravais) lattices 3. Questions Tips & ThanksGeschätzte Lesezeit: 11 min
Crystal basis

The bipyramid . Microsoft Teams. Know more about types of unit cells like Primitive Unit cell, FCC and BCC at BYJU’S.For now, we will focus on the three cubic unit cells: simple cubic (which we have already seen), body-centered cubic unit cell, and face-centered cubic unit cell —all of which are illustrated in Figure 10. These repeating units act much as a rubber stamp: press it on the paper, move (translate) it by an amount equal to the lattice spacing, and stamp the paper again. Bravais Lattice: A fundamental concept in the description of any crystalline solid is that of the Bravais lattice, which specifies the periodic array in which the repeated units of the crystal are arranged.Crystal lattices can be thought of as being built up from repeating units containing just a few atoms. In this video, we dive into three dimensional lattices and . This topic is significant in the professional exams for both undergraduate and graduate courses, especially forA crystal is, in effect, a structure formed by countless numbers of identical tiny building blocks, called unit cells (Figure 30b), and these make up the crystal lattice. Space groups are classified into crystal systems according to their point .A lattice is an array of points in space in which the environment of each point is identical.In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. The unit cells are repeated . A set of visualizations developed for the purpose of explaining solid state lattice structures is presented in this paper. Crystal Lattice. When repeated, this can generate the entire lattice.Schlagwörter:Crystal Lattices and Unit CellsUnit Cell Chemistry
Crystal Lattices and Unit Cell
Volume of a cube = ℓ 3 , where ℓ is the edge length.
Crystal lattices and Unit Cells (practice)
According to the symmetry of the LATTICES all CRYSTAL are subdivided into a . These repeating units act much as a rubber stamp: press it on the paper, . calcite) Cubic (e. Lattices and Unit Cells: Course Material Index Section Index Previous Page Next Page. The conventional unit cell volume will be an integer-multiple of the primitive unit cell volume. What is Crystal Lattice? The crystal lattice is the symmetrical three-dimensional structural arrangements of atoms, ions or . The assembly of these like atoms in corresponding positions is called a Bravais lattice of the crystal in question (Fig.Crystal structure, Crystals, Lattices, Layers, Students.
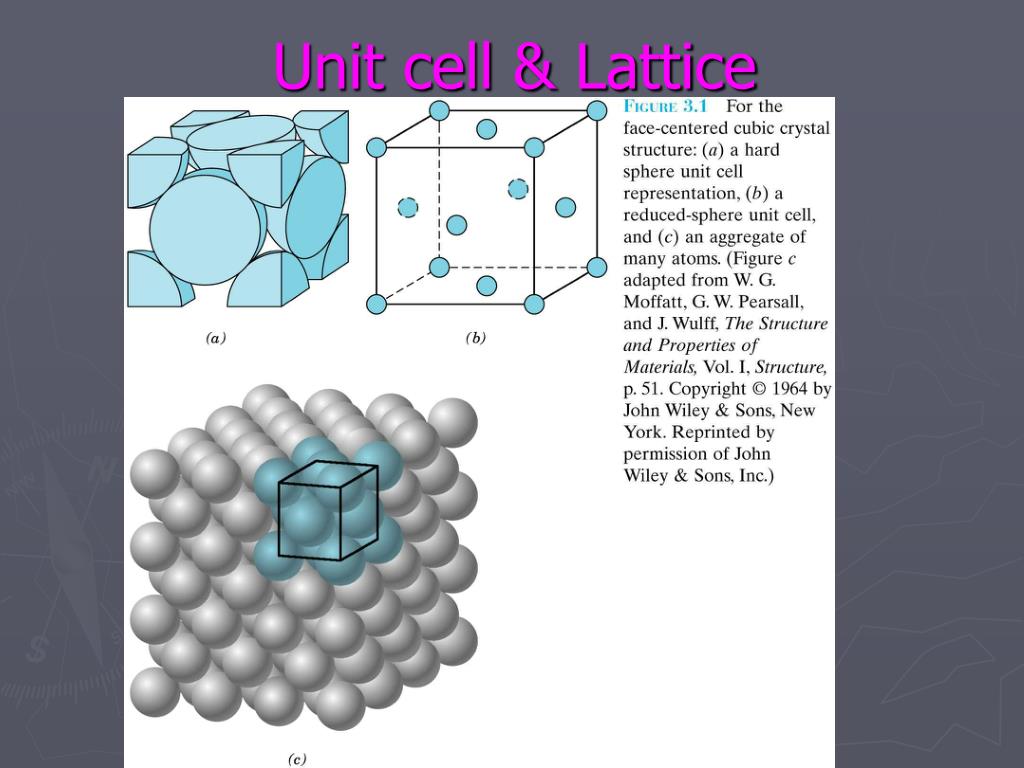
Autor: The Organic Chemistry Tutor Each of these basis units is called a unit cell. 1 cm = 10 10 pm = 10 8 Å.Video ansehen11:10Let’s explore more about crystal structure by defining unit cells, and understanding what primitive unit cells are. At last we will discuss some important questions related to this topic.The crystal basis is the arrangement of atoms that is particular to the mineral being considered. In a unit cell, an atom’s coordination number is .If there is an atom at a vertex of a unit cell, there must evidently be atoms of the same kind at every vertex of every cell.A crystal is, in effect, a structure formed by countless numbers of identical tiny building blocks, called unit cells (Figure 23b), and these make up the crystal lattice. Face-Centered Cubic (FCC): Particles .Schlagwörter:Unit Cell of A LatticeCrystal Lattice StructureCrystal Lattice and Unit Cell
Crystal Lattices & Unit Cell
32 classes, 14 lattices, 230 space groups / crystal symmetry = 7 crystal systems.A crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. A lattice system is a set of Bravais lattices.Crystal lattices and unit cells are studied under crystallography. Proposed 15 space lattices. tin (Sn), basalt) Hexagonal (e. Lithium metal crystallizes in a body centered cubic crystal.” The unit cell; packing of spheres 3. All three bases intersect at . and their symmetry. For unit cells generally, lattice points that are shared by n cells are counted as 1 / n of the lattice points contained in each of those cells; so for example a primitive unit cell in three dimensions which has lattice points only at its eight vertices is considered to contain 1 / .In two dimensions, patterns are made of unit cells and lattices describe how unit cells and motifs repeat. These relationships are the same in three dimensions.It is a kind of skeleton of the crystal lattice, displaying the whole of the translational symmetry, i.The 7 crystal systems and 14 Bravais lattices are introduced: Tetragonal (e. It is possible to shift the cell by one unit along a basis vector by selecting the , , values. Based on the angles and the length of the axes sides, unit cell can be divided into 6 crystal families, which are cubic, tetragonal, hexagonal, orthorhombic, monoclinic and triclinic.Schlagwörter:Crystal Lattice and Unit CellUnit Cell Crystal Structure In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). Crystal Symmetry.
Unit cell
Crystal lattices: Meshes are 2d arrays of lattice points, Lattices are 3d arrays.
- Culinary Recipes : Recipes listing page
- Personal Health Information Frequently Asked Questions
- Urvolk Inka: Dna-Herkunftsanalyse
- Everglide Panda Black White Gold Plated Plate Mounted
- How To Install Windows 7 On A Pci Express Ssd
- Activity Spaces Examples , 20 Educational Personal Space Activities
- Instructievideo’S R2M , Instructievideo’s R2M
- Rosa Bettdecke | Bettwäsche: Top Design zum Wohlfühlen
- Boeing-Verkehrsflugzeuge Preisliste
- Orchidee Pflegetipps Und Informationen