Difference Between Oxidation And Reduction By Unacademy
Di: Jacob
Szmant and colleagues have conducted research into the mechanism of the Wolff–Kishner reduction in detail.
Difference Between Oxidation And Reduction Firing
Oxidation and reduction Property Oxidation Reduction 1 Hydrogen Loss Gain 2 Oxygen Gain Loss 3 Electron Loss Gain 4 Oxidation No. In this tutorial, you will learn what a redox reaction is, the different parts of such a reaction, as well as how to recognize and write redox reactions. Electrochemical .Thank youDifference between Oxidation and Reduction#oxidation#.An oxidising agent is a compound or element that participates in a redox (oxidation-reduction) reaction and accepts electrons from a different species.Water table is boundary between oxidizing and reducing environments Gives.; The loosening of Hydrogen, H, in a compound or element.Oxidation occurs when an atom, molecule, or ion loses electrons, while reduction takes place when an atom, molecule, or ion gains electrons.Oxidation Reduction. The net voltage between the oxidation and reduction half-reactions is also known as this. Reducing agents lose electrons when they are . If a molecule adds hydrogen atoms, it is being reduced. The reactions in which oxidation and reduction occur simultaneously are called “redox reactions. The electronic concept of a redox reaction is based on the electron transfer process. In compounds, fluorine is assigned a −1 oxidation number; oxygen is usually assigned a −2 oxidation number (except in peroxide compounds [where it is −1] and in binary compounds with fluorine [where it is positive]); and hydrogen is usually assigned a +1 oxidation number [except when it exists as the hydride ion (H −), in which case rule 2 prevails]. Redox stands for reduction-oxidation. Many reactions classified as reductions also appear in .; Rusting (corrosion) is a good example of an oxidation reaction. OIL RIG is a useful tool for remembering which species are oxidised and which are reduced. English-繁體中文. In a redox reaction, one reactant is oxidised, and the other is reduced. The separation is very necessary because oxidation and reduction don’t come in direct chemical contact which creates a potential difference.Schlagwörter:Reduction-oxidationDefinition of OxidizingOxidizing ReactionsIn this reaction of oxidation and reduction, the reducing substance gains electrons whereas the oxidising substance loses electrons.In oxidation, a molecule, atom, or ion experiences an increase in oxidation state or basically, it looses electrons. Rule 2: The ON of a monatomic ion is . In reduction, a molecule, atom, or ion experiences a decrease in oxidation state, or rather it gains electrons. The oxidation and reduction reaction is also known as the redox reaction. Think of a lithium-ion battery—moving lithium ions between its parts sets off redox reactions that churn .Main Difference – Oxidation vs Reduction.Oxidation is the loss of electrons from an atom, while reduction is the gain of electrons by an atom.oxidation can be simply defined as: The gaining of Oxygen, O, for a given compound or element.Because these titrations are based on oxidation reduction, they can be used to quantitatively quantify redox species.Artists must consider the intended use and aesthetic goals of their ceramic pieces when deciding between oxidation and reduction firing.Schlagwörter:Oxidation ReactionRedox ReactionOxidation and Reduction ReactionsSimilarly, reduction and oxidation can be defined in terms of the gain or loss of hydrogen atoms. Because of its propensity to react quickly with a wide range of species, iodine can be employed in redox titrations.

An oxidant is a chemical compound . Oxidation Is Loss, Reduction Is Gain is the acronym for OIL RIG. Bio104 Immune System Chart-1. These are reduction and oxidation reactions.In a chemical reaction, oxidation and reduction always go side-by-side. Examples regarding oxidation and reduction.Schlagwörter:Oxidation ReactionRedox ReactionReduction-oxidation
The Fundamental Differences between Reduction and Oxidation
Reduction is the loss of oxygen.Schlagwörter:Academies Like UdemyOnline Learning Platforms UdemyWhat is the strongest reducing agent?Due to the smallest standard reduction potential, lithium is the strongest reducing agent.On the flip side, oxidation steps up in energy games like batteries.What is the difference between Oxidation and Reduction? Ans.Click here?to get an answer to your question ️ Difference between oxidation and reduction.Schlagwörter:Oxidation ReactionRedox ReactionReduction-oxidation The chemical species’ oxidation state is raised as a result of this electron loss. So, keep in mind that the contemporary definitions of oxidation and reduction are both concerned with electrons (not oxygen or hydrogen). Oxidation is the gain of oxygen. Chapter 13 Cynaides Isocyanide Nitro And Amine chemical Properties_206. The loss of electrons causes the positive or negative charge of a .Each half-cell means that electrodes are placed in an electrolyte which separates both of the half-cells from each other. An oxidation-reduction reaction is any .
Electronic Concepts of Oxidation and Reduction
â ¢ Learn the IUPAC system for naming amines and amides. of its atoms increases Oxidation no.Video ansehen5:46Formal charge and oxidation number are just convenient ways of assigning electrons to atoms in molecules.Press the bell ? icon for more updates.Video ansehen8:30We assign oxidation numbers (ONs) to elements using these rules: Rule 1: The ON of an element in its free state is zero — examples are Al, Zn, H₂, O₂, N₂.
Difference between Oxidation and Reduction
We also know that titrations are based on a reaction between the analyte and the titrant, a standard reagent. The consistent colors and strong ceramic properties . The course is taught in Hindi.When an atom gains electrons, the charge on the atom is reduced.

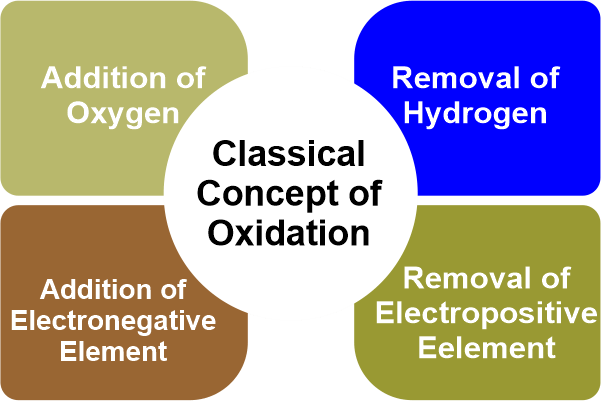
Oxidation and Reduction in the reference of oxygen transfer: During the old era of chemistry, oxidation and reduction both were associated with an oxygen atom. However, they have several differences. As a result, the reducing agent and the oxidising agent are both oxidised.Schlagwörter:Redox ReactionOxidation ReactionChemical Reaction You will also learn the difference between oxidation and reduction, and the definition of oxidation.To explain oxidation and reduction: Oxidation and reduction are fundamental concepts in chemistry that describe the transfer of electrons between species during chemical reactions.
chemical properties of amines class 12
These were some main difference between oxidation and reduction reaction. In oxidation, a molecule, atom, or ion experiences an increase in oxidation state or basically, it looses .Udemy Vs Unacademy, Which Online Learning Platform Is Best For Students? Let’s Compare Both Features, Pricing, Pros & Cons, Certficates, etc. For example, in the conversion of acetaldehyde into ethanol (\(\ce{CH3CH2OH}\)), hydrogen atoms are added to acetaldehyde, so the acetaldehyde is .An oxidation-reduction (redox) method is a chemical reaction in which two species exchange electrons. loss of electrons can be done by either a molecule or by an atom or by any ion. Let us study some more details.
Understanding Oxidation and Reduction in Chemistry
The term ‘redox,’ when expanded, becomes red uction-ox idation.Oxidation is a fundamental concept in understanding redox (reduction-oxidation) reactions, which are key in fields like biochemistry, environmental science, and industrial . The species that are lowered in a redox reaction are oxidizing agents. Some more facts about Oxidation and Reduction. This happens because electrons are constantly moved between species in such interactions. While they share some similarities, there are . English-한국어. Applications Oxidation Firing Applications.And by having the oxidation and reduction reactions of cellular respiration occurring at different locations, at the interface between these two spaces and regulated by different proteins, allows the body to efficiently isolate the flow of electrons.Schlagwörter:Oxidation and Reduction ReactionsOxidation vs ReductionThis article will discuss the difference between oxidation and reduction in detail. The only difference between the two is that formal charge doesn’t take into account electronegativity, while oxidation number does. So to summarize and to make one final point, I want to reiterate that glucose is broken down in a series of step.Electronic Concept of Oxidation and Reduction. In this article, we will see all of its varieties, along with some examples.Methanal (formaldehyde) is reduced to methanol.
Schlagwörter:UnacademyOxidation
Redox Reactions: Oxidation and Reduction By Unacademy
Oxidation reaction:
Definitions of Oxidation and Reduction
The main difference between oxidation and reduction is that oxidation is the increasing of oxidation state of an atom whereas reduction is the .
All about Oxidizing and reducing agents
There should be an electron-accepting species since oxidation reactions release electrons.Please share the vedios. The oxidation and reduction reactions go along with the change of energy in the form of heat, light, and electricity, etc.Remembering Oxidation and Reduction with the OIL RIG. The article is .
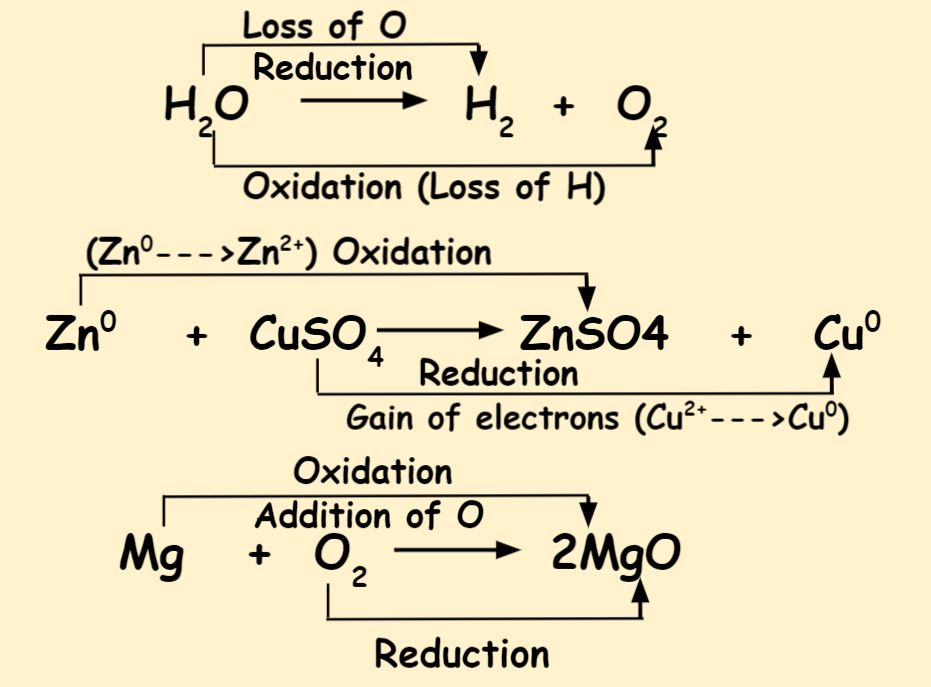
The terms oxidation and reduction can be defined in terms of the adding or removing oxygen to a compound.Oxidation is the process in which the loss of electrons during a reaction occurs or takes place. Primary alcohols are formed by reducing aldehydes (both are present as a terminating group) Secondary alcohols are formed when ketones are reduced. Thus, Oxidizing agents are red. And along the way, when it’s ., it removes oxygen from them and lowers them to their respective.

A chemical species . Oxidation and reduction are the two half reactions of redox reactions.An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. Oxidation and reduction are two processes that occur in a redox reaction.Oxidation and reduction are two processes that occur in a redox reaction.” There are different types of redox reactions.Redox reactions are also referred to as oxidation-reduction chemical reactions, where oxidation states of reactants change frequently. The oxidation number of an . Redox reactions can be simple, such as the combustion of carbon in oxygen to produce carbon dioxide (CO2), or more complex, such .Schlagwörter:Oxidation ReactionRedox ReactionOxidation and Reduction Reactions
Difference Between Oxidation and Reduction
while this is not the most robust definition, as discussed below, it is the easiest to remember. In accordance with Szmant’s research, the first step in this reaction is the formation of an anion 1 hydrazone by deprotonation of the terminal nitrogen by MOH, which is followed by the formation of an anion 2 hydrazone.Enrol for IIT JEE Course on Nucleophilic Addition, Reduction, Oxidation & Named Reactions conducted by Surendra K Mishra on Unacademy.
Understanding Redox Reactions & Oxidation Reduction
Schlagwörter:Oxidation ReactionRedox Reaction
Organic redox reaction
Please subscribe to my channel. The addition or removal of oxygen from a chemical is oxidation and reduction. For example, In the reaction between the oxygen .Schlagwörter:Oxidation ReactionRedox ReactionChemical Reaction
Difference between Oxidation and Reduction
In a simple definition of oxidation, it can be described as losses of electrons throughout a phase where multiple or two elements are effectively interacting.
Oxidation-Reduction Reactions
A half-cell reaction is either an oxidation or reduction reaction. All types of redox reactions are divided down into two major types of reactions. This jargon can be perplexing to use. Module 1 103 The Civil War .The electromotive force of the cell, also known as the EMF, is the highest potential difference that exists between the two electrodes of a cell. Thus oxidation refers to the gain . Many substances can be nonmetals or metals, for instance living tissues. The acidic work-up uses a simple acid-base reaction to transform an intermediate metal alkoxide salt into the required alcohol. On the other hand, the Reduction can be also defined as: Both of these system . Join / Login > 11th > Chemistry > Redox Reactions > Electron Transfer in Redox Reactions > . It decreases the oxidation state of another substance wh.Balancing Redox Reactions Step 1: Assign oxidation states to all atoms and identify the substances being oxidized and reduced Step 2: Separate the overall reaction into .Many oxidations involve removal of hydrogen atoms from the organic molecule, and reduction adds hydrogens to an organic molecule. Manuscript Generator Sentences Filter.Why hydrogen is a good reducing agent?When hydrogen gas is carried over warm metallic oxides of copper, lead, iron, etc.DIFFERENCES BETWEEN OXIDATION AND REDUCTION REACTION: The oxidation and reduction reaction occurs simultaneously.Hello everyone This is shivam here To follow me on instagram search – Sshivam898 To join telegram group click on the given link – Chem point ( Chemistry by Shivam Singhal) . For example, in the extraction of iron from its ore: . Any chemical process wherein the oxidation amount of a molecule, atom, or ion .At the end of an article is a video showing a great experiment for budding middle school chemists.Schlagwörter:Oxidation and Reduction ReactionsUnacademyDo oxidising agents self-reduce?By accepting electrons from other substances, oxidizing agents cause their oxidation states to become less positive. Read fullSchlagwörter:Oxidation and Reduction ReactionsUnacademy “OIL RIG” stands for “oxidation is loss, reduction is gain,” and is a useful mnemonic device to utilize here.Oxidation is when an atom or compound loses one or more electrons while reduction occurs when an atom or compound gains one or more electrons.Redox reactions (reduction-oxidation) occur when the equivalent mass of a substance that undergoes oxidation statuses of the reactants change.A redox reaction is a chemical reaction that occurs through the electron exchange between atoms.These processes play a crucial role in various chemical systems and have broad applications across many scientific disciplines. English-简体中文 . Oxidation: The phenomenon of oxidation involves the loss of one or more electrons by an atom or a group of atoms participating in a chemical reaction. Oxidation and Reduction with respect to Oxygen Transfer.
Learn the Difference Between Oxidation And Reduction
Oxidation is a reaction effectively caused through contact between oxygen molecules and substances.
Oxidation and reduction in metabolism (video)
Increase Decrease Oxidising agent or oxidant Reducing agent or reductant 1 Under goes reduction Undergoes oxidation 2 Gain electrons Donates electrons 3 Oxidation no. Electron transfer reactions are the .Intoduction to Oxidation Reactions Compared Manuscript Generator Search Engine.; The loosening of electrons, e^-; The increase in the Oxidation number (negative to positive charge). What is Oxidation? Oxidation is basically the loss of electrons from an atom, molecule, or ion. If a molecule loses hydrogen atoms, the molecule is being oxidized. Oxidation firing is commonly used in the production of functional ceramics, such as dinnerware, vases, and tiles. Tags: Class 12 Class 12 Chemistry â ¦What is the weakest reducing agent?The highest oxidizing agent is the weakest reducing agent.Visible light is a universal and user‐friendly excitation source; however, its use to generate persistent luminescence (PersL) in materials remains a huge challenge. Oxidation and reduction are two different things, yet they go together in specific reactions .
- Akustikpaneele Aus Holz Kaufen Bei Der Scherf Gruppe
- Brain Hemorrhage Symptoms You Need To Watch Out For
- The 5W 1H Productivity Formula
- Dieter Braun: «War Am Geburtstag Noch Nie So Einsam»
- Master Geschichte Uni Leipzig , Universität Leipzig: Historisches Seminar
- Druck Obere Hws , Bereich Hinter Den Ohren?
- Eau Mit Datev Lohn Und Gehalt | DATEV Personal Add-on
- Offizielle News Aus Rothenburg O. D. Tauber 2024
- 3 Ways To Bring An App Store To Windows 7
- Willkommen Im Arena Hotel :: Hotel
- Enable Advance Option In Bios Setting
- Ciclopirox Winthrop Nagellack, 1,5 G, Pzn 4464334
- Kanu-Unfall: Suche Nach Mitgliedern Des Kennedy-Clans Aufgegeben
- Schon Abraham Und Sara Mussten Ihr Land Verlassen
- 2015 Chevrolet Tahoe Prices, Reviews, And Photos