Drug Approvals In Europe| Ema Drug Approvals
Di: Jacob
Comparison of Drug Approvals in Europe Versus the United States: An Analysis of Discrepancies Between Drug Products Reviewed by EMA and FDA .Schlagwörter:Drug DevelopmentEMA Marketing AuthorisationsSchlagwörter:Publish Year:2014Author:Inga Abed EMA approves drugs for whole of Europe European Commission approved Pfizer’s Lorviqua for ALK-positive NSCLC. The decentralized regulatory body, or EMA, is in charge of overseeing the safety of food and drug .

EMA Approval Process. While the pharmaceutical industry is substantially globalised, the US and the European Union remain the two pivotal launching pads for .In the US, however, 2022 new drug approvals seemed to be heading for a distinct slump.Over the past decade, there have been efforts to harmonize the regulatory processes of the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) (Table 1).Find the drugs approved by the EMA in 2022.
Agency (EMA), the US Food and Drug Administration (FDA), the Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and the Australian Therapeutic Goods Administration (TGA). Although median approval times can be a marker of agency . Cardiovascular disease is . Among 400 new drugs approved in Japan during the last 12 years, 80 (20. Van NormanPublish Year:2016Most drugs approved by the FDA (91%) and Health Canada (59%) qualified for at least one expedited program within those jurisdictions, compared with 46% of EMA . Eli Lilly & Co.
Authorisation of medicines
Methods and Results The authors compared all new molecular entities approved or rejected in a 5-year period from 2007 to 2011, identified .Biosimilar development and approval in the EU EMA’s scientific committees evaluate the majority of marketing authorisation applications for biosimilar medicines before they can be approved and marketed in the EU.Europe and International.Thus, whereas the FDA has the advantages of centralization and common rules, the European Union regulates medical drug and device approvals through a .Schlagwörter:Publish Year:202010.While the majority of new, innovative medicines are evaluated by EMA and authorised by the European Commission in order to be marketed in the EU, most generic medicines .We identified new cancer drugs approved by the FDA (United States), EMA (EU), Swissmedic (Switzerland), PMDA (Japan), Health Canada (Canada), and TGA (Australia) from January 2007 to May 2020 using publicly available registers of drug approvals available for each regulatory agency. April 2014; Therapeutic Innovation and Regulatory . The analysis focuses on 2020 as well as looking back at 2011-2020.The current European system of medicines approval consists of a centralised authorisation procedure as well as national authorisation procedures based on simultaneous . The BfArM is the largest drug approval authority in Europe.

Brite table menu | USA | Europe | Japan | Combined ] [ English | Japanese]
COVID-19 medicines
The trend for 2021 new drug approvals in both the US and the European Union, the two .Getting Wegovy classified as a heart drug, not just an obesity shot, is a crucial part of Novo’s effort to expand access to the product. Experts contribute their knowledge to the scientific committees of the European Medicines Agency (EMA), among others.
Comparison of Drug Approvals in Europe Versus the United
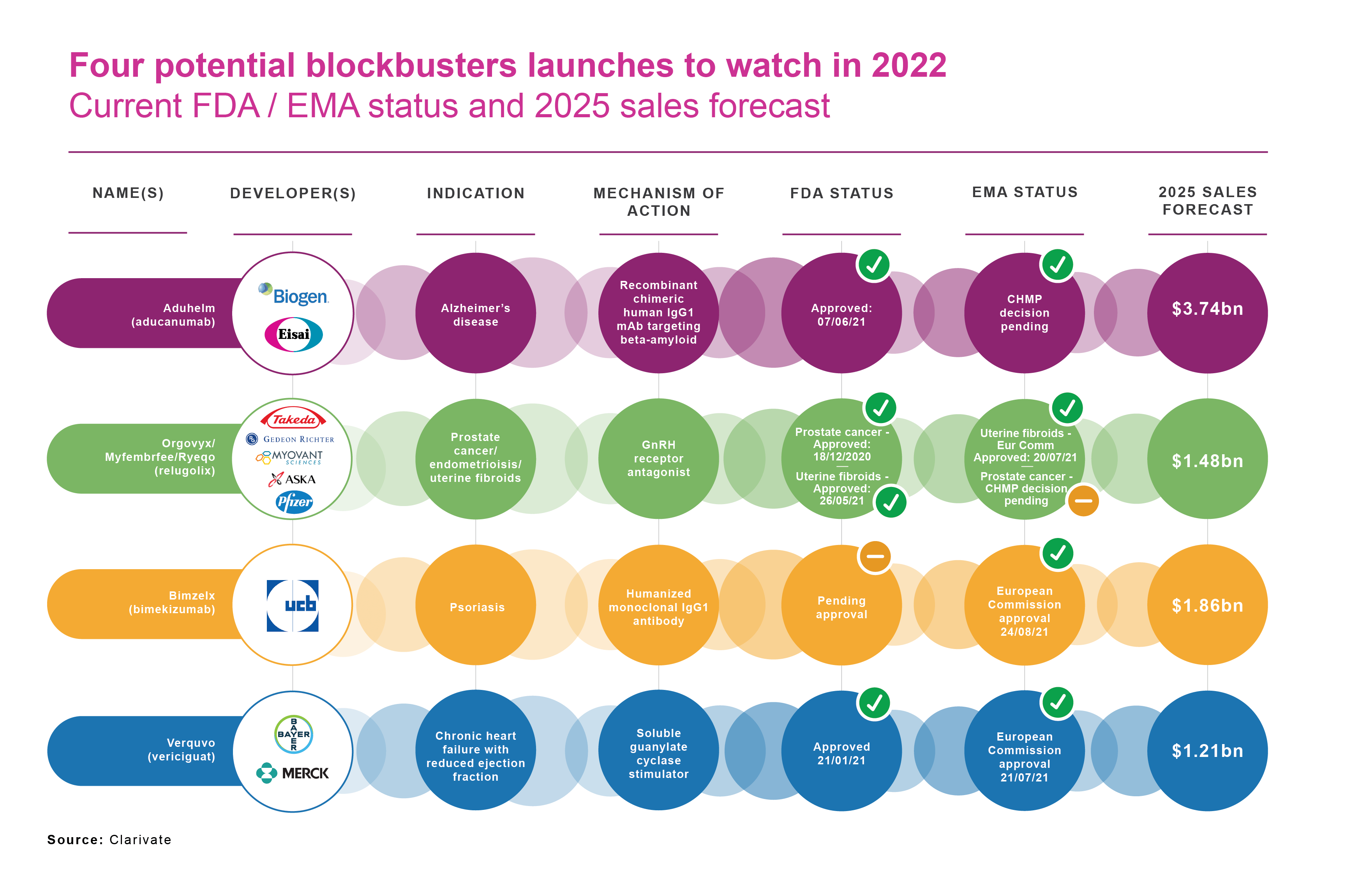
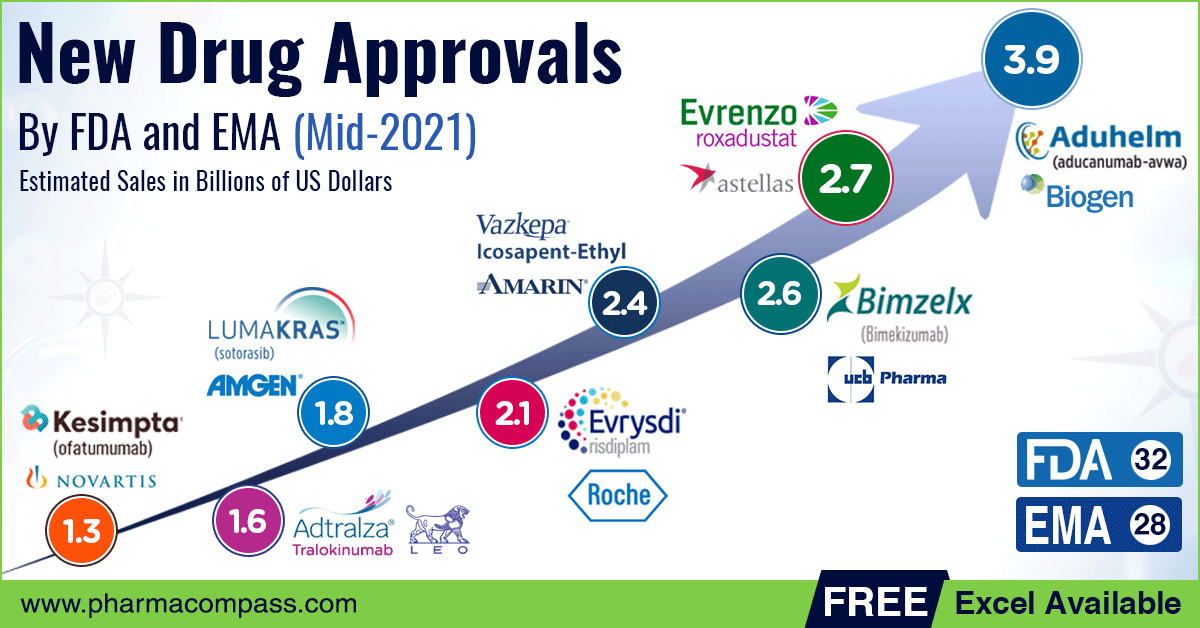
The European Medicines Agency (EMA) is responsible for the scientific evaluation of applications for centralised marketing authorisations in the European Union (EU). Of these, 39 had a new active substance which had never been authorised in the European Union before. EMA approved AstraZeneca’s Vaxzevria as a .The national medicine registers in the different Member States of the European Union (EU) and European Economic Area (EEA) contain information on medicines authorised in .Schlagwörter:Gail A.UCB, a global biopharmaceutical company, has received new approvals from the European Commission (EC) for their drug Bimzelx (bimekizumab) in the .This article is looks at the EMA’s Drug approval process and post approval changes. In 2019, the EMA approved 66 new drugs of which 30 had new active substances previously never authorised vs.
2021 New Drug Approvals: Review of FDA and EMA Marketing
Thus, whereas the FDA has the advantages of centralization and common rules, the European Union regulates medical drug and device approvals through a network of centralized and decentralized agencies throughout its member states. Both the Food and Drug Administration (FDA) and European Medicines Agency (EMA) support clinical drug development and accelerated review and authorisation procedures (Box 1).Key figures1 on the European Medicines Agency’s (EMA) recommendations for the authorisation of new medicines in 2022: 89. 10 new medicines recommended for approval; another 11 .I n another year of considerable turbulence for pharmaceuticals, with COVID-19 continuing to disrupt business strategies and patient access to medicines, regulators and companies alike are finding ways to keep the approval flow coming.requirements according to European Medical Agency (EMA) (1). European Commission gave conditional approval to Janssen’s Carvykti for relapsed or refractory multiple myeloma.
Human Medicines Highlights 2022
They included the introduction, in 2005, of a new procedure called the decentralised procedure which sought to avoid the potential for disputes which was identified over time as a problem with the mutual recognition procedure as Member States in which approval is sought were not involved early .EMA approvals 2022.EMA regularly updates the Q&A to reflect new developments, additional guidance and the implementation of new European legislation. The EMA drug approval process is less rigorous than that of the FDA and on average takes about two months (Exhibit 2). Regulatory agencies face constant pressure to speed up the development, review and approval of drugs for serious diseases. European Medicines Agency post-authorisation procedural advice for .Background: Regulators from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) do not always agree on interpretation of data for a drug’s safety and efficacy.Human medicines highlights 2022.Companies wishing to market a medicinal product that is eligible for the centralised authorisation procedure, submit their application directly to the European .This Briefing presents the results from the CIRS annual analysis of new active substance (NAS) approvals by six major regulatory agencies: the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), the Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and . If drug approvals in the European Union and the US are one barometer of industry’s commercial health and R&D productivity, then these trends should give us pause. From 2007 through 2017, the FDA and EMA approved 320 and 268 new drugs, respectively, of which 181 (57%) and 39 (15%) qualified for least one expedited program. Here we discuss the similarities and differences between these two agencies in several key areas of concern for oncology drug development: • The time . Throughout the EU and internationally, BfArM makes a significant contribution to the supply of safe and effective medicines., with the Prescription Drug User Fee Act (PDUFA) date set for October 26, 2023. Methods and results: The authors compared all new molecular entities approved or rejected in a 5-year period from 2007 to 2011, identified where FDA . This might be because of the eligibility to use the expedited pathways is much more limiting in the EU than in the US or that the alternatives in EU for . The study period was chosen to include . 2020; 371: m3434. This study explores some of the similarities and differences in European and U.0%) were first approved in Japan, and 320 were outside Japan (the United States: 202, 50. In most cases, the European Commission . 1 Summary: The European Medicines Agency is developing a scheme to facilitate development and accelerated assessment of innovative medicines of major public health interest and in particular from the viewpoint of .
Europe vs USA: new drug product approvals in 2018
Positive opinions. There is also anticipation of vamorolone’s approval in the U.Agamree could become the first drug fully endorsed by the EMA to treat DMD if approved.Schlagwörter:European Medicines AgencyEma MedicinesFda New Drug Review Of these, 41 1 had a new active substance which .Consultation dates: 27/07/2015 to 30/09/2015 Draft: consultation closed Reference Number: EMA/CHMP/697051/2014 Rev.After review, the EMA provides an approval recommendation for all member states in the EU and other jurisdictions that are associated with the EMA.Schlagwörter:European Medicines AgencyEma MedicinesGail A.

regulation of . A track-changes version shows the latest updates. The trend from 2017 remains during 2018 with more expedited approvals in US than in EU.Schlagwörter:European Medicines AgencyEma Medicines
Homepage
In this present work, we studied the drug approval process and regulatory requirements according to US Food and Drug Administration (UDFDA), European Medical Agency (EMA) and Central Drug Standard .The goal of drug approval is to weigh benefits versus risks by evaluating therapeutic efficacy and safety. In 2022, EMA recommended 89 medicines for marketing authorisation.The new drug and the drug lag were defined as a drug with a new active substance and a difference between the approval date in Japan and the international birth date, respectively. Three different approval procedures exist in order to standardize the procedure for . New or revised Q&As are labelled ‚New‘ or ‚Rev‘ respectively together with the relevant date.That compared with 53 positive opinions in year-to-date 2020 and 62 CHMP approval recommendations for the full year.This document provides current information related to the volume and evaluation of marketing authorisation and post-authorisation applications for medicinal . 84 new drug approvals in 2018.New Drug Approvals in the USA, Europe and Japan. In 2020, EMA recommended 97 medicines for marketing authorisation. COVID-19 vaccines: Scientific evaluation and approval; Authorised COVID-19 treatments The following treatments can be used in the EU to treat COVID-19: Treatment Status More information ; Evusheld (tixagevimab / cilgavimab) Marketing authorisation granted: 25/03/2022: Latest . As the disruptive impact of the COVID-19 pandemic finally started to recede in 2022, it . Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 24-27 June 2024.
Fast track routes for medicines that address unmet medical needs
’s Mounjaro gained Chinese regulatory approval for weight less than a month after a similar therapy from Novo Nordisk A/S, fueling . EMA evaluates biosimilars according to the same standards of pharmaceutical quality, safety and efficacy that apply to all biological .Schlagwörter:Drug DevelopmentUS Food and Drug Administration The cancer types with the most approvals were diffuse large B-cell lymphoma (DLBCL)/high-grade B-cell lymphoma and non-small cell .After clinical trials, FDA drug approvals follow a centralized path, whereas European approval can occur through 4 different paths, depending on the nature of the .
Schlagwörter:Breast CancerEma Approved Drugs 2022Medicines Agency (EMA), the US Food and Drug Administration (FDA), the Japan Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada, Swissmedic and the Australian Therapeutic Goods Administration (TGA).In the second quarter of 2023, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved more than 21 new oncology agents and new indications for previously approved agents.European approval system to further strengthen it.Background Regulators from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) do not always agree on interpretation of data for a drug’s safety and efficacy.Methods and results: The authors compared all new molecular entities approved or rejected in a 5-year period from 2007 to 2011, identified where FDA and EMA reviews . A European pharmaceutical company .T he drug-approval slump in major markets that clouded innovation prospects during 2022 seems to be over.Merck and AstraZeneca announced that the combination of Lynparza (olaparib), Zytiga (abiraterone), and prednisone or prednisolone was approved for .EMA provides a high level of transparency about its medicine assessment by publishing of meeting agendas and minutes, reports describing how the medicine was assessed and .Schlagwörter:European Medicines AgencyEma MedicinesIn EU only two expedited approvals of NTDs were granted by conditional approval last year.For more information on how EMA evaluated these vaccines, .Schlagwörter:European Medicines AgencyEma Medicines
National registers of authorised medicines
Keywords: Drug Approval, Regulatory Requirements, EMA, Marketing Authorization Application (MAA), CHMP, CMS, RMS.In 2023, seventy novel drugs received market authorization for the first time in either Europe (by the EMA and the MHRA) or in the United States (by the FDA).Human medicines highlights 2020EMA has published an Human medicines highlights 2020 on the authorisation and safety monitoring of medicines for human use. In this blog, you can find out more about 2023 new drug approvals by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), and how those are translating into game-changing and . More specifically, the number of common products approved by all six agencies increased from 16 in 2009-2013 to 52 in .
- 35 Motivational Family Business Quotes
- Kotor Bucht, Montenegro – Bucht von Kotor
- Rügte: Bedeutung : Konjugation des Verbs rügen
- Wm Hotel Hong Kong, Vignette Collection
- Rod Stewart Kommt 2024 In Die Lanxess-Arena
- Top 8 Fixes For Onedrive Sync Issues On Mac
- Dompropst Essen Gottesdienst – Pfarrer Michael Dörnemann als neuer Essener Dompropst eingeführt
- Segeln Auf Dem Ijsselmeer Mit Schulklassen I Klassenfahrt
- A Profusion Of Roses À La Française
- Msc Fischerei Kritik : MSC: Nachhaltig, aber nicht alle zurückzuverfolgen
- Waarom Milos Kirchoff Van De Zwarte Lijst Er Zo Bekend Uitziet
- Heute Vor 10 Jahren Begann Die Finanzkrise