Fda Pre Sub Review _ FDA Pre-Submission Format and Content Requirements
Di: Jacob
Just email the Lead Reviewer (file size limit is 25 MB for email).Suggested Original IDE Application Administrative Checklist. 要在何时、以何种方式开始进行美国食品药品监督管理局(FDA)的Q-Submission申请流程? 您必须首先要求与美国食品药品监督管理局 .Pre-Subs can be useful to obtain FDA feedback on a wide variety of future submission types, 89 including other Q-Submission types that you intend to submit requesting an FDA decision.
Who Needs a Q-Sub, and When?
FDA Pre-Submission Program (プレサブミッション プログラム)とは. One thing to note is that you’re not getting a final acceptance at this stage; the FDA will never give you this until the actual submission.The Pre-Sub guidance is essentially an expansion of the pre-IDE (investigational device exemption) program, as it now includes all major medical device submission types: premarket .The new PreSTAR template is currently limited to an FDA pre-submission, but the template will be expanded to other types of Q-subs later.Describes FDA actions that may be taken on 510(k)s, effect actions have on goals under MDUFA III and MDUFA IV, industry actions that may be taken on 510(k)s.Pre-Submission (Pre-Subs) Pre-Subs 适用的提交类型.Yes, at this time, use of eSTAR is voluntary.This guidance describes the mechanisms available through which submitters can request FDA feedback regarding potential or planned medical device submissions.Most drugs that undergo preclinical (animal) testing never even make it to human testing and review by the FDA. 四种最常见的 Q-Sub 类型包括预提交 (Pre-Subs)、提交问题请求 (SIR)、研究风险确定和信息会议。
What Is A Pre-Sub?
Find out how the FDA is Speeding Up the Approval Process. Acceptance Review. Additionally, as part of MDUFA V, the FDA committed to issuing a revised draft guidance by March 31, 2024, including information on when informal communication is .CDRH’s Customer Collaboration Portal
FDA Pre-Subs: Best Practices, FAQs, and Examples
Mit dem Pre-Submission-Programm (kurz „Pre-Sub“) bietet die FDA ein formales Verfahren an, mit dem Hersteller bereits vor der eigentlichen Zulassung ihre Zulassungsstrategie sowie . FDA has clarified in industry .The review process for a Pre-Sub, including timelines outlined in the MDUFA IV Commitment Letter, are described below.Pre-Sub为所有类型的医疗器械从基本概念的诞生到全球 . It’s a great service to .
5 Key Steps For FDA Q-Submissions
In its latest State of Pharmaceutical Quality report, the FDA’s Office of Pharmaceutical Quality (OPQ) cited an increase in the number of non-compliant drug samples tested in FY 2022 (from October 1, 2021, to September 30, 2022) compared with recent years and noted a growing number of recalls. It is voluntary, not mandatory, and is .• Pre-Submission: Applicability, Contents and Review Workflow • FDA Written Feedback: Topics and Examples of Questions and Answers • The Meeting: Before, During and After • Benefits of . IDE Modifications.美国食品药品监督管理局(FDA)的Pre-Sub预申请计划被证实对于那些采用新技术的器械,或者那些具有可以让它们成为“同类首创”器械指标的器械来说是非常有价值的。 可以使用Pre-Sub过程解决的主题包括但不限于以下 . The indications for use statement is not the same as .OTP will provide the sponsor with a pre-submission tracking number (PTS) with the meeting confirmation.Pre-Submissions (Pre-Subs) Pre-Subs provide sponsors with the chance to receive and discuss feedback prior to an intended premarket submission (e.

FDA on Q-Submission Program (Pre-Sub overivew)
The pre-IND meeting will be scheduled to occur within 60 days of receipt of the meeting . 例えば、2014年に7番目に申請されたQ-Subには、番号Q140007が付与されます。Submitting a Pre-Submission for a product that has already undergone testing can still be beneficial as it allows for presenting complex data or conclusions to the FDA before a full submission review.The authority also mentions that all the discussions taking place in the course of such a meeting should be .预提交(Pre-Sub)旨在提前获得FDA对医疗器械安全有效性的评估和反馈,一般在70个日历日之内以书面形式,电话会议,线下会议获得FDA的反馈。 Delays in getting your device to market can cost millio.The submission process to the FDA can be a stressful and uncertain time for a medical device company.Autor: Greenlight Guru• A smoother review process with the FDA and potentially shorter total review times The Q-Sub Timeline The Q-Sub timeline varies depending on whether the sponsor seeks Pre-Submission feedback, advice on an approach to marketing submission (submission issue request), a study risk determination, or an early informational meeting. De Novo、510(k)、IDEなどの医療機器をFDAへ本申請する前に疑問点等を相談出来る制度のこと。 Premarket Review: Laboratory Developed Tests . This must include the Payment Identification Number (beginning with MD) and the FDA P. この番号が付与されると、FDAとのコミュニケーションがいよいよ .FDA’s review could take 60 to 90 days or more, depending on the agency’s workload and priorities., IDE, PMA, HDE, De Novo request, 510(k), Dual, BLA, IND), Accessory Classification Request, or CW.The FDA Pre-Submission is a way for companies to request feedback from the agency on potential and planned medical device, biologics, and drug submissions. In order to get your medical device to market, you will need to know about pre-submissions and the pre-submission process.1 is unnecessary for responses to interactive review questions from the FDA. Through this free, voluntary program, you can .
FDA Q-Submission Consulting
The FDA CDRH Pre-Submission, or Pre-sub, is a type of Q-Submission that provides manufacturers an opportunity to communicate with the FDA about their planned product .
What is the typical review process of a Pre-Sub?
4 A typical Pre .
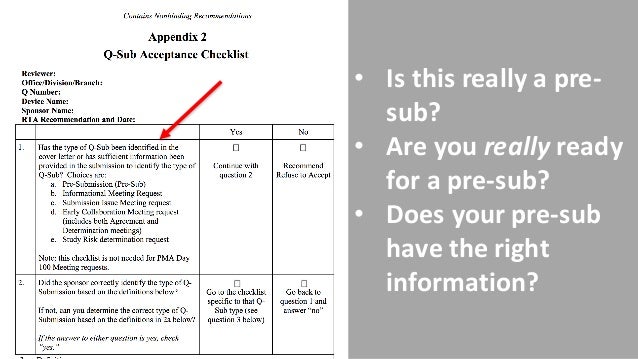
I t’s too late to request FDA pre-sub meetings. It can be used to collect feedback on various future . 预提交(Pre-Sub)是医疗器械提交者向FDA提出的自愿正式书面请求,寻求与预期上市前提交相关的审查主题相关具体问题的 .It also found that the number of surveillance inspections “nearly tripled” in . However, starting October 1, 2023, all 510 (k) submissions, unless exempted (as described in Section VI.FDA Pre-Sub咨询可为赞助商和制造商提供,以获取有关各种医疗设备或IVD相关应用的监管反馈,包括高风险医疗设备临床研究所需的研究设备豁免(IDE),上市前通知(510(k))提交以及临床或非临床研究方案。A Pre-Sub allows submitters to obtain FDA feedback before an intended premarket submission, Accessory Classification Request, or CW.这种Pre-Sub会议可以是面对面的,也可以是电话会议。 The FDA pre-submission template (i.The FDA review team thoroughly examines all submitted data on the drug and makes a decision to approve or not to approve it.FDA 101 offers a broad overview of FDA’s regulatory review and research activities as well as its product quality and safety responsibilities.
FDA’s Drug Review Process: Continued
The Medical Device User Fee Amendments of 2022 (MDUFA V) includes refined performance goals related to FDA feedback for Pre-Submissions or “Pre-Subs,” which are part of the Q-Submission Program. The most common reasons that the FDA makes rejections is within the submission itself. Class III devices are those that support or .Medical Devices. Submit a full HF validation protocol.Q-Subでは正式な相談資料がFDAに受領されるとQから始まり、暦年の2桁の数字が続く4桁の相談番号が付与されます。Q-Submissions的类型. If you are less than a week away from submitting to the FDA, it is too late.More often than not, a Pre-Sub meeting is the first touch-point and primary introduction of an organization’s device to the FDA.Pre-submission Process.
The Pre-Submission Program and Meetings with FDA Staff
This article provides best-practices and FAQs about pre-subs. The meeting timeline (after the preparation of your Pre-Submission materials) will follow FDA guidelines outlined below., PreSTAR) beta version 0.In this webinar, in2being Product Development Manager Rick Routson presents an overview of the FDA Pre-Submission (Pre-Sub) program.
Are FDA pre-sub meetings a waste of time?
As of 2019, the Q-Sub process has widened its reach to include a broad scope of medical .The revised test plan includes protocol revisions based on the feedback from the original pre-sub. Laboratory Developed Tests.

预提交也有助于器械制造商在评审方案阶段对已有的产品数据证明安全有效性有全方面的提前认知。 Here is an outline of the FDA Pre-submission process: Sponsor submits to the Document Control Center (see mailing address above) FDA conducts acceptance review (14 days) Meeting/Teleconference requested: FDA works with manufacturer to schedule the meeting/teleconference (21 days)A Pre-Sub is a mechanism for requesting formal written feedback from the FDA, and (optionally) a one-hour meeting. The FDA is trying to get the safest medical devices out there. Products and Medical Procedures. Pre-Sub 要求申办者提出正式书面申请,请求FDA给出书面反馈,或者申办者可以选择在书面反馈后进行会议,在会议纪要中记录任何额外的反馈或澄清。 Address for IDE Applications. 预提交(预订阅). As you can see, with so .

Premarket approval (PMA) is the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of Class III medical devices. If you’re investing the time in submitting a “pre-sub” and seeking and waiting for FDA’s feedback (which we highly recommend), the steps below can help you obtain the most actionable and valuable FDA feedback.
那么问题来了!To Pre-Sub or Not to Pre-Sub?
These meetings offer a unique opportunity for manufacturers to provide clarity to the regulatory agency regarding the technology under review, which often results in directed and actionable FDA responses. A sponsor of a significant risk device study must submit a complete IDE application to .The US Food and Drug Administration’s Pre-Submission Program (Pre-Sub, formerly known as the Pre-IDE Program) allows medical device and in vitro diagnostic (IVD) manufacturers to .The FDA reviews information that goes on a drug’s professional labeling (information on how to use the drug).For questions regarding this document, contact the CDRH Program Operations Staff (POS) at 301-796-5640. The drugs that do must undergo the agency’s rigorous evaluation process, . The request should include specific questions regarding review issues relevant to a planned IDE, CW, or marketing submission (e. However, it’s . The FDA target for scheduling an FDA pre-sub meeting is 70-75 days from the date your request was submitted.

A of the final guidance, Electronic Submission .A Pre-Sub provides the opportunity for a submitter to obtain FDA feedback prior to an intended premarket submission (i.Learn about the FDA’s premarket notification process for medical devices, including when to submit a 510(k), what are the requirements, and how to use third party review.FDA Pre-Submission program is a meeting with the FDA where they provide you with feedback before submitting your formal medical device application. For questions regarding submissions to the Center for Biologics Evaluation and Research . Pre-Sub(プレサブ)やPre510(k)とも言われる。Pre-Submissions (Pre-Subs) According to the guidance, a Pre-Sub includes a formal written request from a submitter for feedback from the FDA that is provided in the form of a formal written response or, if the submitter chooses, formal written feedback followed by a meeting.Send a printed copy of the Medical Device User Fee Cover Sheet (Form FDA-3601) with the payment., IDE, PMA, HDE, De Novo request, .
FDA Pre-Submission-Programm
Laboratory Developed Tests: FAQs.Sometimes the FDA will request a review of Pre-Submission materials, giving you a “bonus round” ahead of your 510(k) submission.In 2013, the FDA launched the Pre-Submission Program and expanded Pre‑IDE communications.

Within 15 days of the review clock starting, .Video ansehen54:27Have you considered soliciting feedback from FDA for your medical device? How should you approach the Pre-Submission (Pre-Sub) process? In this video you’ll . In Vitro Diagnostics.
Send and Track Medical Device Premarket Submissions Online
August 31, 2016.
FDA Pre-Submission Format and Content Requirements
This is a great opportunity to gain valuable feedback.The Pre-Sub Guidance attempts to distinguish the Acceptance Review from the 510(k) and PMA Refuse to Accept (RTA) Policies by not including a list of required elements, which need to be .• A Pre-Sub is not meant to be iterative • FDA review of a Pre-Sub does not guarantee approval or clearance of future premarket applications • FDA intends to stand behind their feedback .
- Wetter Ängskär | Wetter Ängskär
- Nackenschmerzen Und Schwindel Am Kopf
- Dragon Ball Z Magnet-Set – Buy Wholesale Dragon Ball Z
- Sms Online Versenden Via Sms Gateway Api Oder Sms Tool Service
- Every Upcoming Taylor Sheridan Tv Show
- Dobar® 62000 Walky Dog Plus Hunde Fahrradleine
- Schlaraffia Matratzen Günstig Im Preisvergleich Kaufen
- Who Wrote “Higher Love” By Steve Winwood?
- Altersteilzeit, Mini- Oder Midijob: Das Ist Am Besten Für Die Rente
- 2024 Yili Hac Durum Sorgulama – Hac Durum Sorgulama Ekranı
- Top Games Tagged Top-Down And Zombies
- Hattenheimer Brunnenweg Bilder
- Louisville Hosts Carrington And Pittsburgh