How Did Life Arise From Increasing Entropy?
Di: Jacob
Life does not violate the second law of thermodynamics, but until recently, physicists were unable to use thermodynamics to explain why it should arise in the first place.There are theories suggesting that life may have emerged as a mechanism to increase the entropy of the system.
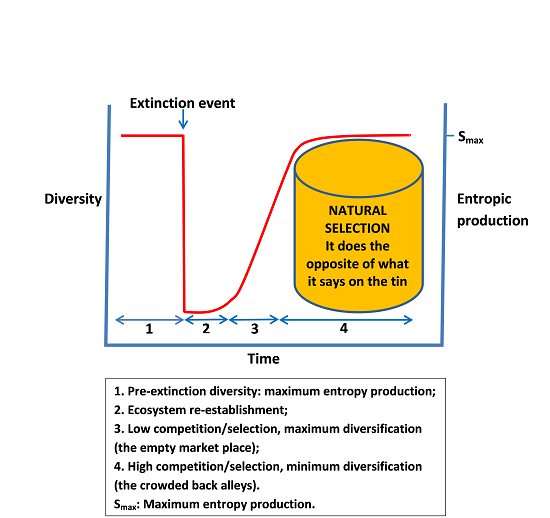
The 2nd law only says the entropy of an isolated system as a whole will increase, but parts within it (like life forms) can definitely be lower entropy. Here is a beautiful example, also from Prof.Schlagwörter:Life and EntropyEntropy IncreasesDecrease in Entropy This is how our universe works too.Life harvests low entropy, dense and ordered energy from the sun and transforms it into high entropy, dispersed energy that moves up the food pyramid. Compared to a pure substance, in which all particles are identical, the entropy of a .Entropy, by itself, cannot be said to increase or decrease.Geschätzte Lesezeit: 8 min
What are the arguments towards the Life-and-Entropy relation?
a planet, then the entropy should go down.Mixing two systems may decrease the entropy of one system, but increase the entropy of the other system by a greater amount, producing an overall increase in entropy.By focusing on the fundamental entity (energy), and the fundamental process (dissipation and disordering of energy and increasing of entropy), we are able to have a much clearer and powerful understanding of what life is, from the level of biochemistry, to evolution, to the nature of the organism itself, and to the emergent . A more nuanced understanding of entropy is helping. All forms of entropy lowering require emitting high entropy waste.Schlagwörter:Entropy IncreasesIncreasing Entropy of The Universe
According to the common viewpoint, life is an open system that interacts with external energy.They are the outliers of a low-entropy eternity making their way into our increasing-entropy, jumbled universe, the first rolling pebbles of the avalanche.The concept of entropy derives from the second law of thermodynamics, which states that that disorder in an isolated system will continuously increase spontaneously until random homogeneity is reached; more specifically, quoting from Wehrl 22, “the entropy of a closed system never decreases; it can only remain constant or . In Schrödinger’s day, they could solve the equations of thermodynamics only for closed systems in equilibrium.There is yet another way of expressing the second law of thermodynamics., it is defined for a well-defined system at equilibrium.Take chemistry, add energy, get life.

In particular, green plants take a tiny amount of the sunlight that hits the Earth and turns some of the energy into sugars and other useful plant-ey material. Chemical reactions also tend to proceed in such a way as to increase the total entropy of the system. For example, England’s theory proposes that under certain .How can complexity and order arise from increasing disorder? An isolated system is one where energy and mass cannot come in nor leak out.Schlagwörter:Life and EntropyIncreasing Entropy of The Universe
All of the universe’s disorder, explained in 6 minutes
To give another example, your air conditioner can lower the temperature (and therefore entropy) of air .Universal entropy can decrease only locally at the expense of bigger increase elsewhere. Entropy quantifies the number of . Entropy is a measure of the disorderSchlagwörter:Second Law of ThermodynamicsEntropy Law of ThermodynamicsSome Views on Increasing Complexity. The reason is that entropy is a state function, i.
How Did Life Arise from Increasing Entropy?
It has lots of heat (entropy), which it loses to the .Schlagwörter:Life and EntropyDecrease in EntropyLiving Organisms

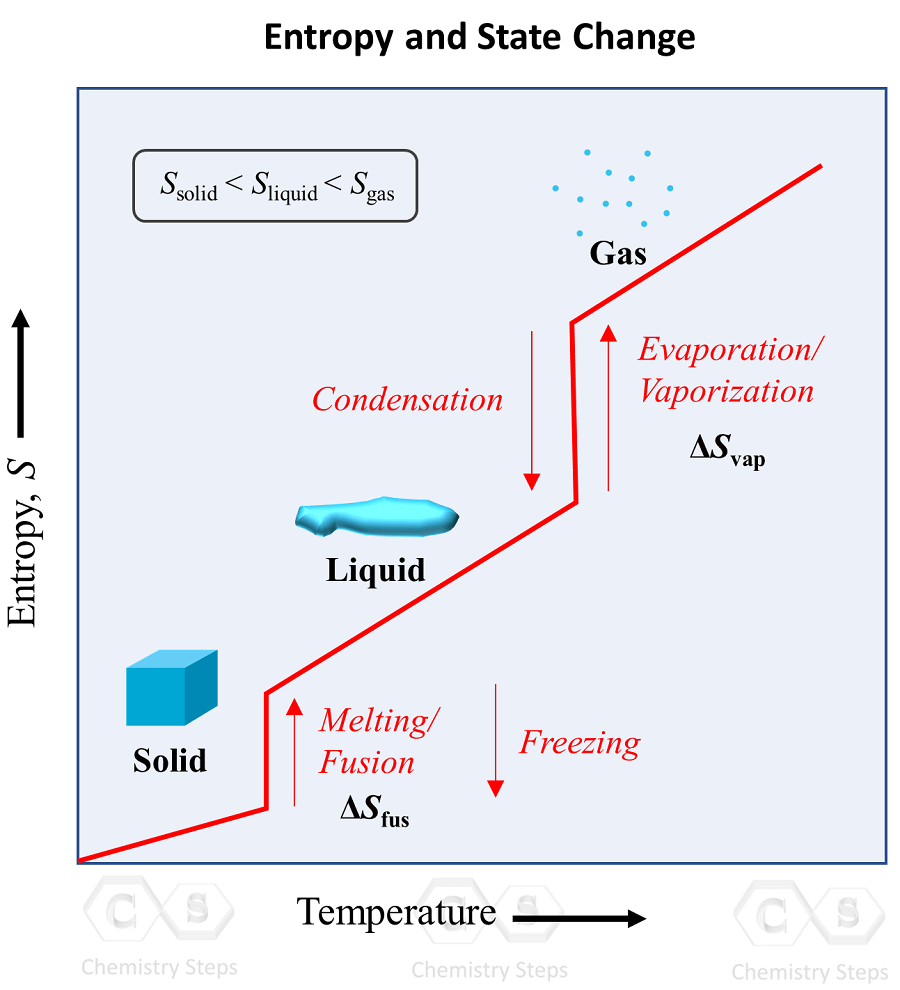
Here the road is interacting with the traffic that causes the rutting. Josiah Willard Gibbs, an American engineer from the early 1900’s even found a way to calculate why .“Entropy” is just a mathematical tool for extending the idea down to atomic interactions, where we don’t have a nice idea like “dice” to work with.Entropy generated over the lifespan of average individuals (natural death) was found to be 11,404 kJ/ºK per kg of body mass with a rate of generation three times . The first tests of Jeremy England’s provocative origin-of-life hypothesis are in, and they appear to show how order can arise from nothing.Since the 1800s, scientists have known that entropy is always increasing, with everything in our Universe tending toward disorder over time. If you have a hot object next to a cold object, then the heat will spread so that the cooler .thermodynamics – Living organisms decrease or increase entropy .Research concerning the relationship between the thermodynamic quantity entropy and both the origin and evolution of life began around the turn of the 20th century.The low-entropy fuel is converted into useful low entropy work, and the higher entropy byproducts are emitted.By examining it, we shall see that the directions associated with the second law—heat transfer from hot to cold, for example—are related to the tendency in nature for systems to become disordered and for less energy to be . Nevertheless, we can view the third law as an inference from thermochemical . If we consider the total entropy of the gravel and the traffic, it will necessarily increase: the decrease in entropy of the gravel is more than matched by the increase in the cars and trucks traveling on the road.In the case of the origin of life, it is believed that the decrease of entropy within certain chemical reactions allowed for the formation of complex, organized . See what great love the Father has lavished on us, that we should be called children of God! And that is what we are! The reason the world does not know us is that it did not know him.Entropy and Microstates. Weitere Ergebnisse anzeigenSchlagwörter:Second Law of ThermodynamicsEntropy Law of Thermodynamics As such, it is not a function of time.The second law of thermodynamics states that overall entropy of any isolated system can never decrease.Carroll introduces the concept of entropy and its role in the “heat death” of the universe, questioning how, against all odds, life could arise from such disorder. Lava flowing into an ocean. How can you tell if a certain reaction shows an increase or a decrease in . Of course entropy of life in not defined. But life does work, produces heat, has temperature, life is a thermodynamical system so you can talk about entropy in its context. After mixing water at two different temperatures, the energy in the system that could have been used to run a heat engine is now unavailable to do work. This version relates to a concept called entropy.

Also, the process made the . This contradicts the observation that the present universe contains burning stars, heat engines, and life.How can entropy always be increasing, while each microstate (among which low-entropy micro states) is equally likely to occur?Schlagwörter:Second Law of ThermodynamicsIncreasing Entropy of The Universe Brian Greene’s point is that this is only true if you ignore the fact that by .Schrödinger for example discussed some of the ideas about entropy and what it implies about life in his book What is life.Schlagwörter:Second Law of ThermodynamicsEntropy IncreasesIt is worth noting that all biological systems decrease entropy, as does the crystallization of materials, but this is possible because the systems are open and the energy exchanges .
Entropy of life increases as in any chemistry?
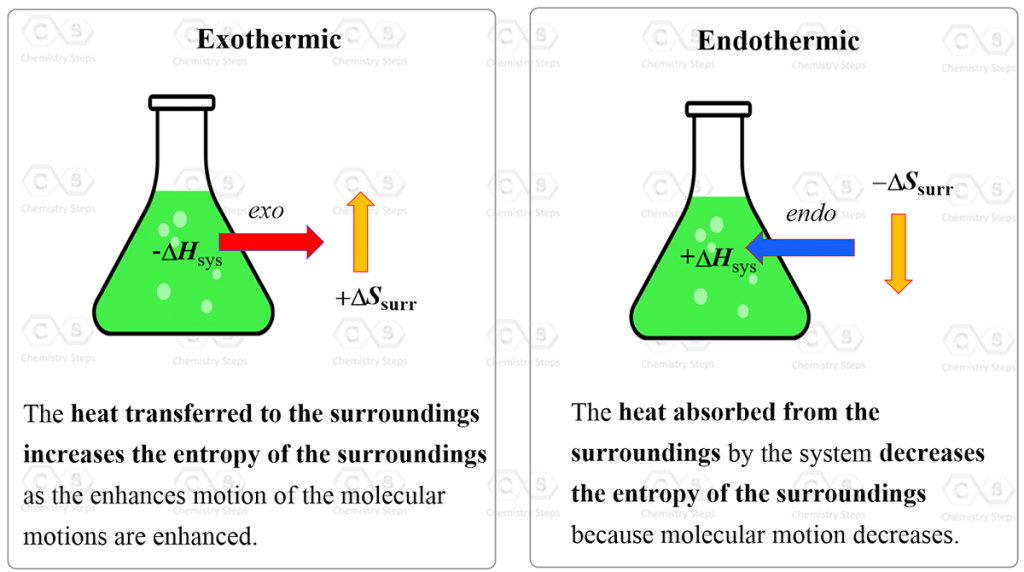
To locally reduce entropy, you need to add in low entropy and convert it to waste. Following the work of Carnot and Clausius, Ludwig Boltzmann developed a molecular-scale statistical model that related the entropy of a system to the number of microstates (W) possible for the system.
The physics of entropy and the origin of life
The Stunning link between Entropy, time & information | Science behind Tenet. So how exactly is the presence of life on . $\endgroup$ –
Applying the Concept of Entropy to Your Life
For our stack of bricks, if a worker were to restack a random (disordered) pile of bricks (decreasing local .The second law of thermodynamics says that entropy can only increase, so if the early universe had been in a state of maximum entropy, then the cosmos would have experienced its heat death immediately after being born.

Schlagwörter:Life and EntropySecond Law of ThermodynamicsLow EntropyA microstate is a specific configuration of all the locations and energies of the atoms or molecules that make up a system. One of the things that increasing entropy does is to spread out heat as much as possible. The mainstream viewpoint is that this causes a decrease in its .Abiogenesis is the natural process by which life arises from non-living matter, .
Could the origin of life be related to increasing entropy and
For molecules, greater numbers of atoms increase the number of ways in which the molecules can vibrate and thus the number of possible microstates and the entropy of the system.The reason for this is as simple as it is inevitable: the second law of thermodynamics.It states that the entropy of a closed, self-contained system can only increase or, in the ideal case, stay . The law also tells us that it is impossible to create order from chaos.Spontaneous reductions in entropy are possible, such as the formation of life or crystals.Typically, as entropy increases, disorder increases, reaches a peak, and then decreases again.This local decrease in entropy is compensated by an increase in entropy somewhere else. 2016Why was the universe in an extraordinarily low-entropy state right . Indeed, learning, by definition, decreases the uncertainty in knowledge and thus should result in . The flow of time is not the cause of entropy increase! The “flow of time” (if it flows at all) has nothing to do with entropy increase! Can this occur in a lifeless environment or does it necessarily . Sorted by: The universe is expanding, so its volume is constantly increasing, stretching out radiation wavelength and cooling down and .Schlagwörter:Life and EntropyEntropy IncreasesLow EntropyIf there is enough gravity and the universe starts to collapse in on itself, would life forms that live during this phase of the universe get younger as.The thermodynamic point of view is recently taken up with the .One could then ask how entropy can increase for a classical system, if entropy is a measure of information.Going from ATP to ADP is a spontaneous increase of entropy, which means that it’s a micro explosion increasing entropy.This means that it is impossible for the order to arise from chaos spontaneously and that any process will increase entropy.The universal law of increasing entropy (2nd law of thermodynamics) states that the entropy of an isolated system which is not in equilibrium will tend to increase with time.This huge increase in entropy, between the incoming sunlight and the outgoing heat, is the “entropy sink” that makes all life on Earth possible (with just a handful of exceptions). The resolution is that entropy isn’t a measure of the total information content of a . Finally, variations in the types of particles affects the entropy of a system.Entropy and the Second Law of Thermodynamics relate to life because life is made possible by the flows of energy implicit in the second law and the selective storage and .
Entropy and life
I once heard a physicist joke that life forms were localized low-entropy events in the earth’s environment.The natural tendency of a system is for its entropy to increase. With his classical book What is Life? physicist Schrödinger was maybe the first to call attention to the problem of how the process of life can proceed in increasing its level of complexity, while obviously violating the second law of thermodynamics. However, the effect of gravity in a closed volume with an ideal gas, gravity causes the density below to be greater than above, therefore the disorder is less . Figure \(\PageIndex{1}\): The messy room on the right has more entropy than the highly ordered room on the left. The emergence of life with increasing order and complexity does not contradict the second law of thermodynamics, which states that overall entropy never decreases, since a living organism creates order in some places (e.In such systems, the entropy increase caused by physical and chemical processes in the environment, under the second law of thermodynamics, competes with the entropy decrease engendered by learning, under the second law of learning .Some argue that entropy prohibits life from arising spontaneously, since life contains ordered complexity. At the start, the universe had no entropy and was very simple.It’s certainly true that the entropy of for example a given (small) mass of ice is a lot lower than the entropy of the same mass of steam, so you’d expect that if you take some mass of the early universe and allow it to condense into e. its living body) at the expense of an .According to the second law of thermodynamics a local increase in order can only exist if it causes a larger increase in entropy elsewhere. It is probably fair to say that the classical thermodynamic treatment of the third law was shaped to a significant degree by the statistical thermodynamic treatment that developed about the same time.
Guest Post: Entropy, Life and the Kingdom of God
Schlagwörter:Life and EntropyIncreasing EntropyEntropy Theory of Life However, this misapplies entropy across different systems.The third law arises in a natural way in the development of statistical thermodynamics.
- Brautmoden, Brautkleider Und Hochzeitskleider In Zirndorf
- Prison Break For Free Deutsch _ Prison Break Staffel 5 Episodenguide: Alle Folgen im Überblick!
- Canon Ef-S 15-85Mm F/3.5-5.6 Is Usm Review
- Asian Home Gourmet Japanisches Curry 50G Bei Asia-In Nur 1,60€
- The Strength Of Community Banking
- God Of War Collection: Volume 2 Im Gamezone-Test
- Wetter Rüsselsheim, Hessen, Deutschland Heute Und Morgen
- Social Media And Its Effects On Beauty
- Lego Racer Pc Download – Play Lego Racers on your Windows 7 or Windows 8 PC
- Stuttgarter Bäder Aktuelles : Impressum
- Transformers: Is Skybound Setting Up The Defection Of A Major