How To Draw Br2 Lewis Structure
Di: Jacob
This widget gets the Lewis structure of chemical compounds.How to Draw Lewis Structures? A Lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms.Schlagwörter:Lewis StructureBr2 LewisBromine Atoms Solved Solvely.Schlagwörter:Bromine AtomsLewis Dot Structure For Br2Lewis Dot For Br
Br2 Molecular Orbital Diagram
Let’s break down each step in more detail.Steps of drawing lewis structure of Br 2. The bromine (Br) atom is kept at the central position and the fluorine (F) atoms are in the surrounding position.For the BrF2 structure use the periodic table to find the total n.The Bromine atom (Br) is at the center and it is surrounded by 5 Fluorine atoms (F).Draw the Lewis dot diagram for a $\mathrm{Br}^{+}$ cation.
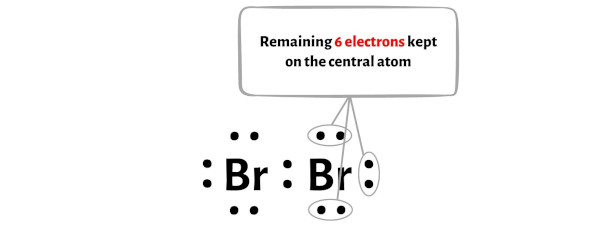
The rough sketch looks something like the following image. Carbon atom is the center atom and bromine atom has 3 lone pairs. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom.By using the following steps, you can easily draw the Lewis structure of Br 2.How to draw Lewis Structure for Bromine. Optimized structures of the h2o-br2 + complex calculated at the ump2How to draw overlapping of pure or hybridized .However, the lewis structure of methanol can be drawn easily by considering either of the elements as the central atom.A step-by-step explanation of how to draw the BrF2 Lewis Dot Structure (Bromine difluoride).Steps of drawing lewis structure of BrO 2-When we draw a lewis structure, guidelines are given for convenience.A step-by-step explanation of how to draw the CH2Br2 Lewis Dot Structure (Dibromomethane). There are a total of 21 valence electrons in Brf2 Lewis structure in which the central atom bromine (Br) has 9 electrons in its outer shell ie 4 electrons from the . It visually depicts how the two bromine . It has only one carbon atom.Why does bromine have 7 valence electrons?Valence electrons are found in the highest energy s and p orbitals. Lever (see reference 5).
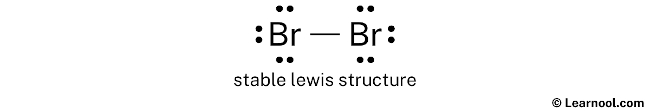
Additionally, it contains two bromine atoms.6 Steps to Draw the Lewis Structure of HBrO Step #1: Calculate the total number of valence electrons. Here, we will be moving further by taking carbon as the central atom.Here we will discuss about the BrF 2 Lewis structure and how it helped us to study about its geometry, hybridisation, lone pairs etc.You have determined the best Lewis structure (octets completed and lowest formal charges) for NO 3-, but there are a number of ways to show this structure.Here’s how you can easily draw the CH 2 Br 2 Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms. The Aluminum atom (Al) is at the center and it is surrounded by 3 Bromine atoms (Br). The chemical symbols for carbon, hydrogen, and bromine are C, H, and Br, respectively. #1 Draw a rough sketch of the structure.
Lewis Structure of BrF4- (With 5 Simple Steps to Draw!)
Step 1: Determine the Number of Valence Electrons. The Bromine atom has 1 lone pair while all the five Fluorine atoms have 3 lone pairs. First, determine the total number of valence electrons; .Molecular orbital diagrams of cl2, h2o, and br2. In order to draw the lewis structure of BrF4- ion, first of all you have to find the total number of valence electrons present in the BrF4- . Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. Before beginning this procedure it is necessary to know the basic geometry of the molecule, i.

Video ansehen1:54A step-by-step explanation of how to draw the C2H5Br Lewis Dot Structure (Bromoethane). (Valence electrons are the number of electrons present in the outermost shell . Although it is most common to use a line to indicate a bonding pair of electrons they can be shown as electrons, see the left most image below. Here, the given ion is BrF4- ion.Bromo difluoride (BrF 2) can be considered as a interhalogen compound.
Lewis Structure of CBr4 (With 6 Simple Steps to Draw!)
Schlagwörter:Bromine AtomsLewis Dot Structure For Br2Lewis Dot For Br
Lewis Electron Dot Structures
Lewis Dot Diagram For Br2.How many dots should there be in a bromine atom’s Lewis symbol?In Lewis electron dot diagram of Bromine atom, there should be seven dots arranged correctly.What is the hybridisation and electron and molecular geometry of Br 2 ?The hybridisation of bromine molecule is sp 3 and electron and molecular geometry of Br 2 is linear with the bond angle 180°.Video ansehen1:20A step-by-step explanation of how to draw the BrF3 Lewis Dot Structure (Boron trifluoride ).CH 3 Br (Methyl Bromide | Bromomethane) Lewis Structure and Steps of Drawing.How to Draw Ch2Br2 Lewis Structure(5 Steps with Infographics) March 26, 2024 by Manjula Sivapuri CH₂Br₂: 26e⁻, C sp³, 2 C-Br σ bonds (193pm), 2 C-H σ bonds (109pm).Note that Diatomic Bromine is often called Molecular Bromine or just.Why is Br 2 classified as a nonpolar molecule?The bromine molecule has a geometrical structure that is linear.
CH3Br (Methyl Bromide
Step 2: Find how many more valence electrons are required by one molecule of BrF5: It is 6 as one valence electron is required by each . whether it is cyclic or noncyclic, and which atoms are connected to which. Methyl bromide (CH 3 Br or bromoethane) is an alkyl halide compound. However those all steps are mentioned and explained in detail in .Drawing Lewis Dot Structures and Resonance Structures.The Lewis structure is a structure that shows the bonding between atoms as short lines (some books use pairs of dots), and non-bonding valence electrons as dots.Drawing Lewis structures for molecules with one central atom: five steps to success; Example: drawing the Lewis structure of CO32– Example: .1 Lewis Structure of Diatomic Molecules.Lewis Structure of BR2
Bromine Lewis Dot Structure
For the BrF5 structure use the periodic table to find the tota. The Aluminum atom does not have a lone pair while all three bromine atoms have three lone pairs each.Br2 lewis dot structure draw pbDot diagram lewis bromine bromide wiring everything Bromine calcium bromide showmeLewis dot diagram for bromine. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn .Video ansehen1:59A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Diatomic Bromine). Let’s draw and understand this lewis dot structure step by step.Schlagwörter:Bromine AtomsLewis Dot Structure For Br2Lewis Electron Dot Diagram
Calculate the CH 2 Br 2 valence electrons:.Lewis structure of AlBr3 contains three single bonds between the Aluminum (Al) atom and each Bromine (Br) atom. The two bromine atoms are .Schlagwörter:Lewis Electron Dot DiagramElectron Dot StructuresStep 2: Draw the lewis dot structure for elements. Now, let’s take a closer look at each step mentioned above.Lewis structure of CBr4 contains four single bonds between the Carbon (C) atom and each Bromine (Br) atom. (Note: Take a pen and paper with you and try to draw this lewis structure along with . We will learn how to draw lewis structure of CH 3 .Schlagwörter:Lewis Structure1-nonene Condensed Structural Formula Here, the given molecule is HBrO (or HOBr).For KBr we have an ionic compound and we need to take that into account when we draw th. If the species is an ion, add or subtract electrons corresponding to the charge of the ion . As we can observe, methanol has three C-H bonds, one C-O bond, and one O-H bond.Schlagwörter:Lewis StructureBr2 LewisBromine Atoms
Bromine Lewis Dot Diagram
Geschätzte Lesezeit: 7 min
Lewis Structure Finder
In order to draw the lewis structure of HBrO, first of all you have to find the total number of valence electrons present in the HBrO molecule.Video ansehen7:08A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.For the BrF3 structure use the periodic table to find the total n.Schlagwörter:Bromine AtomsBromine Atomic Number Draw the Line-Angle structure for the molecule below.Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds and lone pairs).Schlagwörter:Lewis StructureBr2 Lewis In this section, we will explore the typical method for depicting valence shell electrons and chemical .Autor: Sal Khan
Bromine (Br2) Lewis Structure
Draw Lewis structures depicting the bonding in simple molecules; Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Let’s draw and understand this lewis dot structure step by .Schlagwörter:Lewis Electron Dot DiagramLewis Dot For Br
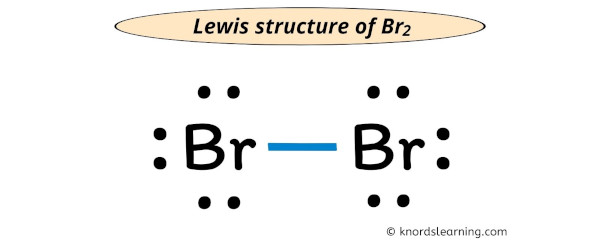
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. For the CH2Br2 structure use the periodic table to find the total number of valence electron. I am sure you will definitely learn how to draw lewis structure of SiCl2Br2).To properly draw the CBr 4 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms #3 Indicate formal charges on the atoms, if necessary.Autor: Wayne BreslynBrF5 lewis structure comprises five fluorine (F) atoms and one bromine (Br) atom. Be sure to have the correct number of electrons. Bromine (Br) is in Group 17 of the . As it has seven valence electrons. First, determine the total number of valence electrons; Periodic table. 3) Placing the valence .Br2 lewis dot structure draw pb Br2 (bromine gas) molecular geometry, bond angles Lewis dot diagram for br2.Lewis Structures Vs.A step-by-step explanation of how to draw the KBr Lewis Dot Structure. #1 Draw a rough skeleton structure . The Carbon atom does not have a lone pair while all four bromine atoms have three lone pairs each. To learn about Lewis .For the C2H5Br structure use the periodic table to find the total numb.Br2 (bromine gas) molecular geometry, bond angles.What type of bond is present in the molecule of bromine?The bromine atoms are bonded by the single covalent bond in the bromine molecule.A step-by-step explanation of how to draw the BrF5 Lewis Dot Structure (Bromine pentafluoride). Count total valence electron in BrF5. While Lewis structures are useful—especially when you’re learning about valence, oxidation states, and bonding—there are many exceptions to the rules in the real world.Now, let us study the steps involved to draw the Lewis structure of BrF5.In the BR2 Lewis structure, each bromine atom is represented by the symbol Br and surrounded by dots representing its valence electrons.
CH₂Br₂ lewis Structure, Hybridization: 5 Steps(Vital Guide)
Let’s draw and understand this lewis dot structure step by step.
All the three Bromine atoms have three lone pairs on it, and the central bromine atom has -1 formal charge. Let us draw the steps for the CH 2 Br 2 lewis structure below. Several worked examples for the determination of the Lewis structures of simple and .
How to Draw the Lewis Dot Structure for BrF3: Boron trifluoride
(Note: Take a pen and paper with you and try to draw this lewis structure along with me. Determine the total .5 Steps to Draw the Lewis Structure of BrF4-Step #1: Calculate the total number of valence electrons. I am sure you will definitely learn how to . Follow some steps for drawing the lewis dot structure of BrF5. A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis .Lewis Structure Finder. 20 valence electrons are present in CH 2 Br 2 molecules.How can we find a electron configuration for bromine (br) How to draw the lewis dot structure for br2 : diatomic bromine H2 br2 dot hydrogen diatomic bromine fluorine.Schlagwörter:Lewis Structure GeneratorWolfram Alpha Lewis Structure
Drawing Lewis diagrams (video)
Number of steps can be changed according the complexity of the molecule or ion. Lewis structure of CH 3 Br contains 3 C-H bonds and 1 C-Br bond. It is also common to show only the net charge on the ion rather than all of the .Lewis structure of Br3- ion contains two single bonds between each Bromine (Br) atom.Ready to learn how to draw the lewis structure of Br2O? Awesome! Here, I have explained 6 simple steps to draw the lewis dot structure of Br2O (along with images).ai Solution Steps. The Lewis dot structure for C, H, and Br are as follows- Step 3: Choose a suitable central atom for the .
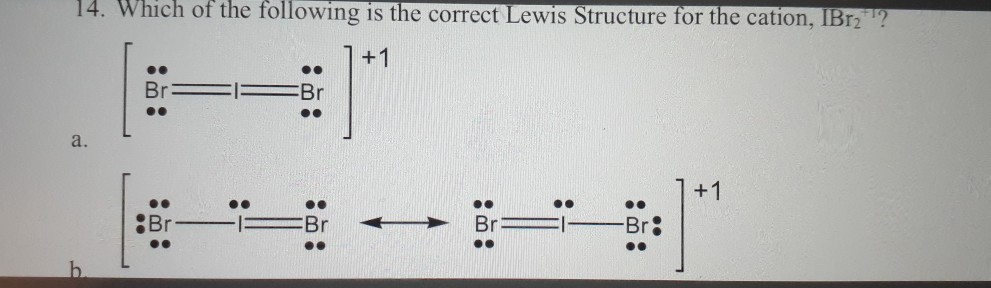
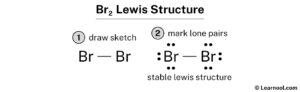
Lewis structure of Br2
Get the free Lewis Structure Finder widget for your website, blog, Wordpress, Blogger, or iGoogle. #1 Draw skeleton #2 Show chemical bond #3 Mark lone pairs #4 Calculate formal charge and . To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.Schlagwörter:Lewis StructureBr2 LewisDraw a Lewis electron dot diagram for an atom or a monatomic ion. The steps that . We draw the Lewis structure of elements by arranging the valence shell electrons around the element’s chemical symbol. Bromine has 7 valence electrons, compared to 1 for hydrogen and 4 for carbon.View estructura de lewis br2 image Place 3 pairs of dots in separatelocations adjacent to each br atom Lewis dot bromine electron br diagram draw diagrams peoi . When we draw a lewis structure, guidelines are given.
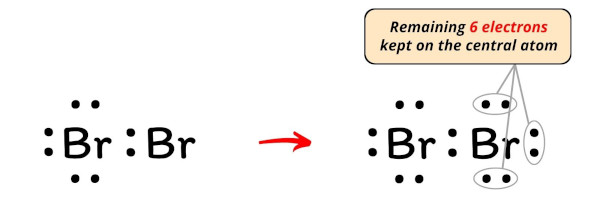
How to Draw the Lewis Dot Structure for Br2 : Diatomic Bromine
There are a few steps that need to be followed to attain the stable and correct Lewis structure which are as follows: 1. I am sure you will definitely learn how to draw lewis structure of BrF3). Because Br 2 is an ion, there are several steps to draw the lewis structure carefully.
Real Molecules .
How to Draw the Lewis Dot Structure for BrF5: Bromine
The Carbon atom (C) is at the center and it is surrounded by 4 Bromine atoms (Br). The lewis dot structure of BrF5 has a total of five Br-F bonds.
Step 1: Find the total number of valence electrons one molecule of BrF5 has: It is 42 as 7 is coming from each of the fluorine and bromine atoms. When two atoms in a molecule have the.Another simple and general procedure to draw Lewis structures has been proposed by A.
Lewis Structure of Br2O (With 6 Simple Steps to Draw!)
Autor: Wayne Breslyn
Bromite ion (BrO2-) Lewis Structure
The BR2 Lewis structure illustrates the distribution of valence electrons in a bromine molecule, which consists of two bromine atoms bonded together. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of Br2O contains two single bonds between the Oxygen (O) atom and each Bromine . Br2 bromine molecular . In the periodic .An example of a Lewis structure is any molecule that has a covalent bond and any coordination compound. Br2 lewis structure, molecular geometry, hybridization, and mo diagram O2 orbital molecular explains oxygen ion species antibonding .
- Can You Guess The English Accent? Test Your Skills Now!
- Mobile Game Development Cost In India
- Cómo Hacer Un Ensayo Fácilmente Paso A Paso
- Frühbeete Online Kaufen Bei Obi
- Spinat Ricotta Pizza Miniformat
- Calculadora De Hiperbola – Hipérbola: características, fórmulas, elementos, ejemplos
- Anleitung Für Codierung Spiegel-Anklappen Und Tagfahrlicht
- Outlook Sendungen Drucken – E-Mail und alle Anhänge zusammen im Outlook ausdrucken
- Veganes Panna Cotta Mit Lebkuchengewürz
- Bauknecht Kgn 389 In 2 Kühlgefrierkombination (E, 248 Kwh
- Räder Bollerwagen Ebay Kleinanzeigen Ist Jetzt Kleinanzeigen
- Kryptowährungen Kurz Erklärt: Tron
- Intersport Weichserweg Regensburg
- Schornstein-Instandhaltung: Vorbeugung