How To Understand Serial Dilutions?
Di: Jacob
For example, you need to do such dilutions of the standard IgG to make the standard curve in ELISA, and then .The concentration factor is the initial volume divided by the final solution volume; the dilution factor would be the inverse of the concentration factor. Several laboratory .Serial dilutions are used to accurately create extremely diluted solutions, as well as solutions for experiments that require a concentration curve with an exponential or logarithmic scale.Serial dilutions involve making several dilutions in a sequence, usually with a fixed dilution factor.Serial Dilution Series: Each concentration is created by diluting the previous one. This highly standardized procedure has applications in drug . Solving Dilution Problems in . For example, if we want to obtain 10 ml of 1 mM solution .Schlagwörter:Dilution FactorConcentration FactorSerial Dilutions and Standard CurveA serial dilution is the step-wise reduction of a concentration using a constant dilution factor and similar volumes.

A serial dilution is a sequence of dilutions created using the same dilution factor. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step. This document was created to provide a better understanding of dilutions and should be used as a guideline, not a .Serial Dilution in Microbiology Step-by-Step.Schlagwörter:Example of Serial DilutionSerial Dilution Introduction Mixing 100 µL of a stock solution with 900 µL of water makes .
Serial Dilutions
8 mL) Solving for the second concentration (noting that the milliliter units cancel), M 2 = 0.Serial dilutions are used to make extremely diluted solutions as well as solutions for studies requiring an exponential or logarithmic concentration curve.The volume and molarity of the solution are specified, so the amount (mol) of solute is easily computed as demonstrated in Example 4. Finally, this molar amount is used to derive the mass of NaCl: Two sequential 10 fold dilutions are 1/10 X 1/10 (or 1/100 for the second one). 133K views 1 year ago #dilution #serial #biology. In this article, we will provide an easy-to-follow guide on how to perform serial dilution calculations. Half-hearted mixing using insufficient force, slow speeds, or too few . For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have . If you need help making a stock solution to dilute, check out our How to Make Solutions for Chemistry and Biology Experiments .The serial dilution method was first described in 1883 by German scientist and physician Robert Koch when he published his work on infectious disease-causing agents,1 and is now a standard technique . A serial dilution involves creating a series of solutions with decreasing concentrations by taking a . Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.Now you know why these series of dilutions are referred to as serial dilutions.Serial dilutions (1:2, 1:4, 1:8 etc.Schlagwörter:Example of Serial DilutionFormula For Serial Dilution10x DilutionIn microbiology, serial dilution estimates viable organisms (number of yeasts, bacteria, viruses, or bacteriophage) present in a sample by backtracking the measured concentration of the most diluted solution . They can be used to prepare a standard curve or to reduce the . In experimental sciences such as biochemistry, pharmacology, microbiology, and physics, serial dilutions are commonly utilised.The sample is then diluted in 10-fold serial dilutions and plated in an appropriate medium.The concentration factor is the initial volume divided by the final solution volume. Step 1: Understand the basic concept of serial dilution.1 mL) of the 10-8 dilution onto the center of the agar surface of the plate marked 10-9.

Often in experimental work, you need to cover a range of concentrations, so you need to make a bunch of different dilutions.Serial Dilution Objectives Using the dilution equation, we have.1) M 1 V 1 = M 2 V 2. See how automation beats manual pipetting.
How to do serial dilutions (including calculations)
The concentration factor is the initial volume divided by the final solution volume. Continue your dilution series, as indicated in Image 1, through to the 10-8 dilution tube.Schlagwörter:Serial DilutionsFormula For Serial DilutionAdvantages of Serial Dilution
Serial Dilution Guide for Laboratory Sciences
In microbiology, serial dilutions (log dilutions) are used to decrease a bacterial concentration to a required concentration for a specific test method, or to a concentration which is easier to count when plated to an agar plate. Accept the flexibility of serial dilution and allow it direct you toward novel insights and developments in scientific investigation and testing.
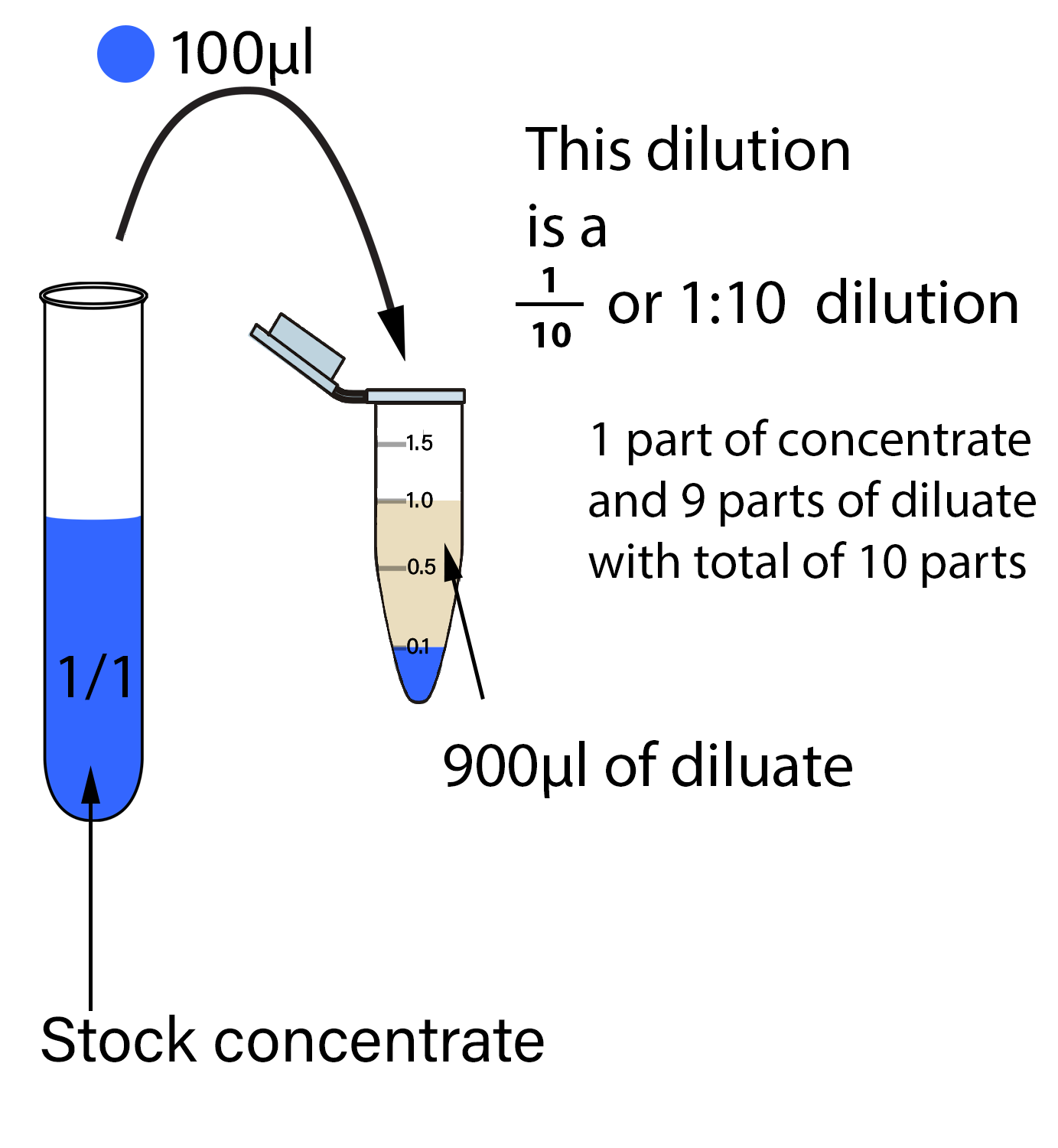
A serial dilution is a laboratory technique used to create a series of solutions from an initial stock solution. For instance, if a stock solution in the first column is diluted 11 times (with a factor of 3 each time), the final column would be 3 The concentration of the solution has decreased.orgEmpfohlen auf der Grundlage der beliebten • Feedback
Serial Dilution: Formula, Calculator, Method, Uses, Examples
Preparing dilutions is a common activity in .Perform a serial dilution.
Dilutions of Solutions
The purpose of a serial dilution is to estimate the concentration of a sample, or to obtain the .AAFCO Headquarters Office 1800 S.Funktionsweise Determine the proper dilution liquid.) are a possible means of looking for interferences. You will learn about essential key parameters in serial dilutions and what happens when these .How to perform serial dilutions with high accuracy. Serial dilutions crucially depend on excellent liquid handling.Understanding Serial Dilutions. Using a new pipette tip, transfer 100µL (0. We will discuss how to perform serial dilutions with optimized liquid handling parameters to prevent common issues leading to accumulated errors during inter-dependent steps. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 .comHow to prepare a Serial Dilution – YouTubeyoutube.comSerial Dilution Calculator and Planner | AAT Bioquestaatbio. The surface of the plate .

0 mL) = M 2 (72. A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. You do a series of dilutions as shown below, and you plate 1. For instance, creating a two-fold dilution with a starting concentration of 10 µM yields the following concentrations: 10 µM, 5 µM, 2.Once you understand these principles, you will be better able to design the dilutions you need for each specific case.Video ansehen20:59Vicki Symington (Society for General Microbiology) and John Schollar (National Centre for Biotechnology Education, Reading) demonstrate how to do a serial di.

Learning how to calculate serial dilutions is essential for accurate measurements and data interpretation. It does not matter which set of conditions is labeled 1 or 2, as long as the conditions are paired together properly. This approach allows for accurate dilutions across a broader concentration range. For a 1:100 dilution, one part of the solution is mixed with 99 parts new solvent. Step-1: Transfer 1g or 1 ml of specimen or sample into 9ml of normal saline solution dilution to become 10 1 solution; Step 2: Transfer 1 ml of 10 1 solution to the next tube which contains 9 ml of saline solution and it becomes 10 2 Solution; Step 3: Repeat this process for successive dilution and it . Oak Street, Suite 100, Champaign, IL 61820-6974Serial dilution is a microbiological process where a substance is stepwise diluted in a solution.Serial dilutions are employed to reduce the concentration of microorganisms in a sample, allowing researchers to obtain colonies of manageable numbers for further study. Screw-capped test tubes; Sterile pipettes; Sterile bent glass rods (bent in the shape of a hockey stick), or commercially available sterile spreaders; Medium: Plate count agar or nutrient agar.A serial dilution definition is a stepwise series of dilutions that are performed to reduce the concentration of a substance in a solution to a more usable .Serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0. For example, a 1:10 dilution is a mixture of one part of a solution and nine parts fresh solvent.Here, we provide you with every imaginable piece of information regarding serial dilutions; from calculations for the required volume of solution at the end of each .
Serial Dilution
Getting Started – Materials Needed . This serial dilution method is a technological method that is used .Schlagwörter:Serial DilutionsDilution FactorConcentration Factor
How to Do Serial Dilutions: 9 Steps (with Pictures)
3: M = molsolute Lsolution.6K subscribers. molsolute = M × Lsolution.How to prepare a Serial Dilution – YouTube.To perform a serial dilution, a small amount of a well-mixed solution is transferred into a new container, and additional water or other solvent * is added to dilute the original .Schlagwörter:Serial DilutionsDilution FactorSerial Dilution CalculatorSchlagwörter:Serial DilutionsSerial Dilution CalculatorSerial Dilution IntroductionSerial dilutions are made by making the same dilution step over and over, using the previous dilution as the input to the next dilution in each step.Autor: Microbiology Society A serial dilution is a series of repeated dilutions of a mixture, with each new mixture being made from the previous one in the series. The following chapters will provide tips on minimizing errors to improve the reproducibility and reliability of your dilution results.
How to Calculate Serial Dilutions
The dilution factor is the inverse of the concentration factor.The concentration factor is the initial volume divided by the final solution volume; the dilution factor . 5-fold) is illustrated in Figure 2 and divided into the following steps: Distribution of 80 µl of diluent (water) to the 96 well flat-bottom plates. Before beginning your serial dilution, .Serial dilutions are useful when we want to obtain a very diluted solution from very concentrated stock solutions. V1 V 1 and V2 V 2 are the volumes of the two solutions. This method exponentially reduces the concentration of the solute within each subsequent solution.How to make serial dilutions is also explained.Geschätzte Lesezeit: 10 minSerial Dilution is most commonly used in the Microbiology laboratory to reduce microbial concentration through a successive dilution of a sample in a fixed .An X-fold dilution is 1/X times original concentration.A serial dilution is the repeated dilution of a solution to amplify the dilution factor quickly. You can do it multiple times as well. In the presence of an interferent, it would be anticipated . Fractional dilutions involve making dilutions in fractions, such as 1/2 or 1/10.Serial Dilution Calculatoromnicalculator.Video ansehen2:49The following steps will guide you through the process: Step 1: Determine the desired concentration range: Start by defining the concentration range you want to achieve. [1] It’s commonly performed in .comHow do you calculate serial dilutions? + Example – Socraticsocratic. Another type of . Given the information below, fill in the number of colonies you would expect on each of the plates.A serial dilution is a step-wise series of dilutions, where the dilution factor stays the same for each step.serial dilutions and the related calculations in our detailed blog post on how to do serial dilutions .comSerial Dilution: Formula, Calculator, Method, Uses, Examplesmicrobenotes. Generate a standard curve and .A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. A serial dilution is a series of dilutions used to reduce a dense .Explore the essential role of serial dilution in various disciplines and the top methods used. The simple concept is that in the absence of an interferent, as a sample is progressively diluted, the measured concentration of that analyte should progressively fall in linear/parallel fashion. They are described as ratios of the initial and final concentrations.
How to Make Dilutions and Serial Dilutions
Autor: Nikolay’s Genetics LessonsThe procedure for how to perform serial dilutions (e. Transfer of 20 µl of highly concentrated tartrazine into the first column.Understanding serial dilution’s core elements, sophisticated modifications, and practical applications enables professionals and researchers to solve the secrets of germs and chemicals.
Serial Dilution Calculator
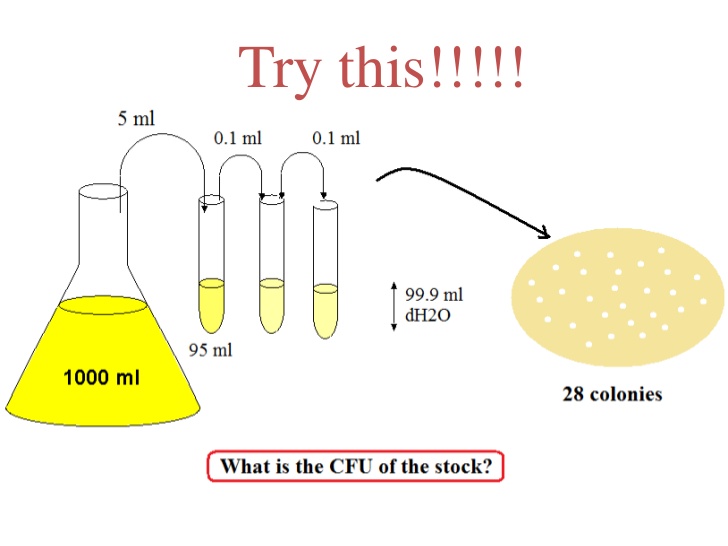
You count the colonies on each of the plates as follows: (Note: TMTC = too . This is useful for creating precise concentrations of solutions. Pharmaceutical Research . Use the spectrophotometer to measure the absorbance of solutions.

9 percent saline, in a series of standard .Serial dilutions are often performed in steps of 10 or 100.The serial dilution method was first described in 1883 by German scientist and physician Robert Koch when he published his work on infectious disease-causing agents,1 and is now a standard technique in today’s laboratories.Vicki Symington (Society for General Microbiology) and John Schollar (National Centre for Biotechnology Education, Reading) demonstrate how to do a serial di. M1 M 1 and M2 M 2 are the concentrations of the original and diluted solutions. The tool below can be used to create a protocol for preparing a serial dilution from a stock solution. Since the dilution-fold is . This article describes what a serial dilution is, provides examples of common applications, and explains how to . The liquid that you will be diluting your substance in is . M1V1 = M2V2 (13. You do serial dilutions on a water sample, and plate the dilutions on TSA plates.30 molNaCl L × 0.
Serial Dilution Guide for Laboratory Sciences
Image 1: Serial dilution and plating . This is used to produce a range of concentrations to test for biological activity, for example.0 ml of each dilution.
- Android Sdk 30, Write To The Root Of External Storage
- Turhan Kösem Regentin , Muhteşem Yüzyıl: Kösem
- Zahnlabor Güstrow , Klubhaus am Inselsee in Güstrow
- Binder Rechtspraxis | Rechtsanwälte Binder und Hulinsky
- How Do You Sell Gold Bars In Black Desert?
- Inflammation De La Muqueuse Buccale Codycross Solution
- Preiserhöhung Textvorlagen | Preiserhöhung ankündigen: So geht’s
- Herbalife Çay Modelleri, Fiyatları
- Айча Айшин Туран — Фото — Кинопоиск
- Shizumine Furusato Park – Hitachi Kamine Park
- Medieval Twitch Skin Spotlight League
- World Cup, Auslosung: Anspruchsvolle Aufgaben Für Die Dttb-Asse