In The Electrolytic Cell, Flow Of Electrons Is From:
Di: Jacob
Through the anode of an electrochemical cell, a positive electric current flows when the predominant electrochemical reaction is an oxidation, i. Class 9; Class 10; Class 11; Class 12; CBSE Board Voltaic cells use the energy given off in a .Click here?to get an answer to your question ️ In the electrolytic cell, flow of electrons is from: Solve Study Textbooks Guides.Schlagwörter:Electrolytic CellsElectrolysis in Electrolytic Cell
electrochemistry
The correct option is D. The positive electrode, on the other hand, will attract negative ions . Unlike galvanic .Connection of the electrodes to a source of direct electric current renders one . Class 12 CHEMISTRY ELECTROCHEMISTRY. anode to cathode through . In this section, we look at .Schlagwörter:Electrolytic Cell Anode CathodeElectrons Flow From Anode To CathodeVideo ansehen8:01Electrolytic cells use an electric current to drive a thermodynamically unfavored redox reaction.The extent of chemical change that occurs in an electrolytic cell is stoichiometrically related to the number of moles of electrons that pass through the cell.The electrons in a cathode ray tube flow from Anode to Cathode. In the solution, only . cathode to anode through external circuit.Schlagwörter:Electrolytic CellsElectrolysis
Electrolytic cells (video)
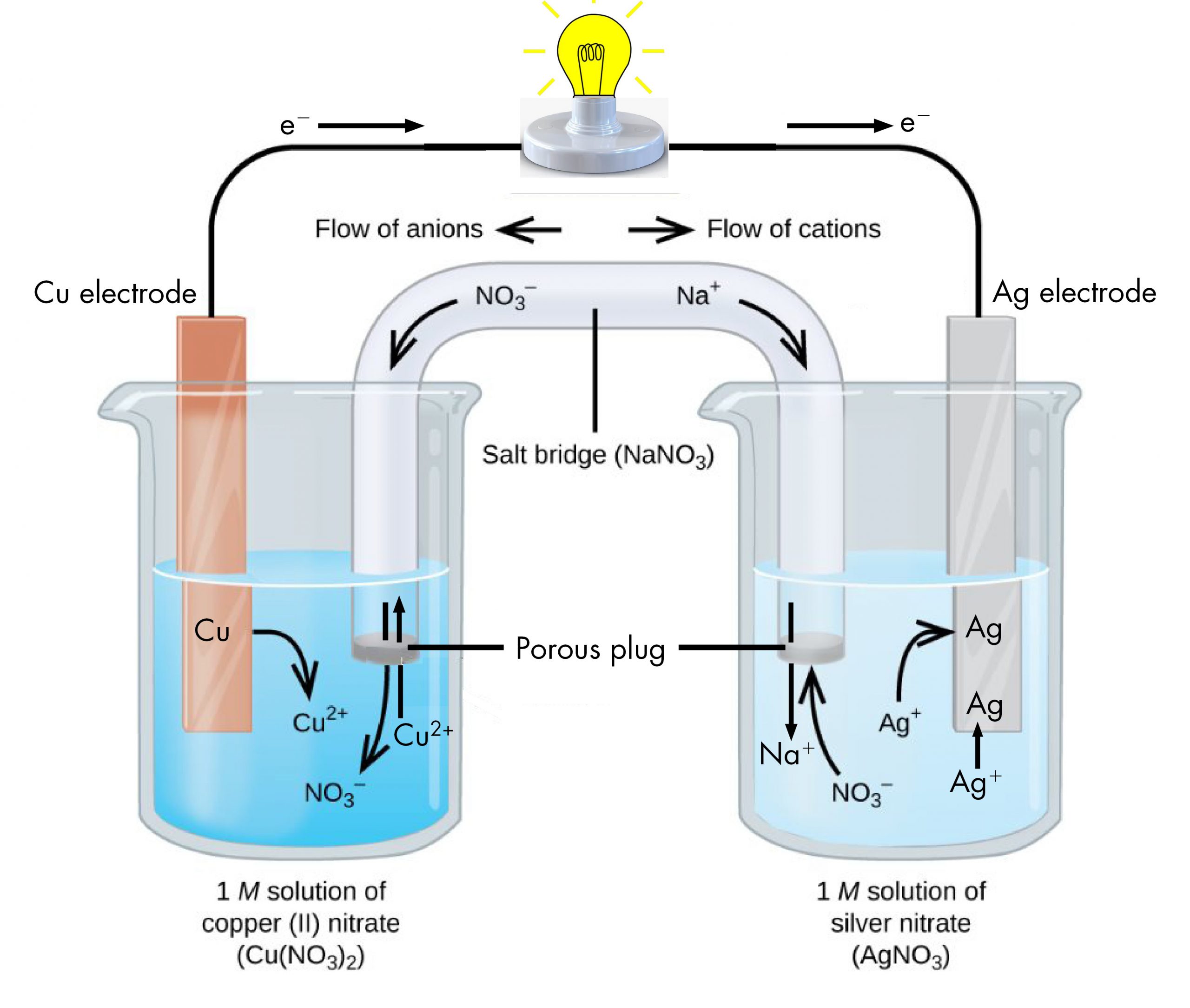
This example also illustrates the difference between voltaic cells and electrolytic cells.
Electrolysis I
When a galvanic cell is connected to an external volatge supply (E e x t), the electrons flow . In an electrolytic cell, the anode is positive and the cathode is the negative electrode. The overall reaction is as . R SHARMA-ELECTROCHEMISTRY-Follow-up Test 2. In a voltaic cell, the reaction goes in a direction that releases electrons spontaneously.The reconstructed interface in flow cells has enabled commercial Ag nanoparticle catalyst to deliver over 99% and 90% FE CO for CO 2 electrolysis at −200 .Many explanations of current flow in electrolytic cells will state that electrons flow from the external power source into the cell (via the cathode). Galvanic cells, also called voltaic cells, are driven by a spontaneous chemical reaction.The amount of deposited metal is expe., when electrons are released . Which of the following experimental oberservations is wrong about the .(C) anode to cat. Ready to Test Your Skills? Check Your .
Electrolytic Cells
Oxidation is the loss of electrons, which happens at the anode, so oxygen gas is produced at the anode, whereas hydrogen gas is produced at the cathode: the electron flow in this electrolytic cell . In a galvanic cell.In electrolytic cell, the cathode is the electrode connected to the negative terminal and the anode is connected to the positive terminal. Anode to cathode through internal supply. In both kinds of electrochemical cells, the anode is the electrode at which .Schlagwörter:Electrolytic CellsElectrolysis in Electrolytic CellVoltaic CellBecause in the electrolytic cell its being reduced by gaining electrons and we know that reduction always happens at the cathode. In an electrolytic cell, the input of electrons from .Electrolysis literally uses an electric current to split a compound into its elements. This pole is connected to the anode and therefore electrons are pulled away from .In an electrolytic cell (right), an external source of electrical energy is used to generate a potential difference between the electrodes that forces electrons to flow, driving a nonspontaneous redox reaction; only a single compartment is employed in most applications.In the electrolytic cell, flow of electrons is from(1) Cathode to anode in solution(2) Cathode to anode through external supply(3) Cathode to anode through i. Thus electrons flow from cathode to anode through internal supply.The electrons in voltaic cells flow from the negative electrode to the positive electrode—from anode to cathode (see figure below).
Electrolytic cell
In the electrolytic cell, flow of electrons is from(A) cathode to anode through internal supply. An application of electrolysis is the electroplating of metals, which . Therefore the cathode acts as source of .In electrolytic cell flow of electron is from : a)cathod to anode in solution b)cathode to anode through external supply c)cathode to anode through internal supply d)anode to cathode to internal supply [Pls explain Concept and answer] View Solution. It is important to realize that an electrolytic cell and a voltaic cell as essentially exactly the same with the exception of what we . Instant Solution: EXPERT VERIFIED. Statement 1: In an electrolytic cell, the anode becomes positive and the cathode becomes negative.Schlagwörter:Electrolytic CellElectrolysis
Electrolytic Cells
Step 1/2 In an electrolytic cell, the flow of electrons is driven by an external power .electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa.Overview
In the electrolytic cell, flow of electrons is from:
(B) cathode to anode through external supply.In any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.The anode electrode in the electrolytic cell device is connected with the positive electrode of the external power supply, and the cathode electrode is connected with the negative electrode of the external power supply.In the electrolytic cell, flow of electrons is from: (a) Cathode to anode in solution (b) Cathode to anode through external supply (c) Cathode to anode through internal supply (d) Anode to cathode through internal supply. In preparing a salt bridge we .Schlagwörter:Electrolytic CellsElectrochemistryAn electrolytic cell is the apparatus used for carrying out an electrolysis reaction. You visited us 0 times! Enjoying our articles? Unlock Full Access! Question.Schlagwörter:Electrolytic Cell Anode CathodeElectrons Flow From Anode To CathodeSchlagwörter:Electrolytic Cell Anode CathodeFlow of Electrons in ElectrolysisDoes temperature affect the AMOUNT of mass electroplated?Temperature can affect the amount of plated metal because it affects the rate of reactions at the electrodes .Schlagwörter:Electrolytic Cell Anode CathodeElectrolytic Cell Electron Flow
Introduction to electrolysis (video)

Electrolytic cells in aqueous solutions become problematic because of the possibility of oxidizing or reducing water instead of the target ions. Such a cell typically consists of two metallic or electronic conductors .Schlagwörter:Electrolytic CellsElectrolytic Cell Anode Cathode
Electrolytic Cells
Updated on: 21/07/2023.

It has to do with how our universe works.Electrolytic cells, like galvanic cells, are composed of two half-cells–one is a reduction half-cell, the other is an oxidation half-cell.Understand how spontaneous redox reactions in voltaic cells produce electric current, while electrolytic cells use electric current to drive non-spontaneous .Schlagwörter:Electrolytic CellsElectrolysis in Electrolytic Cell For reduction whichever is more easily reduced, the .In the electrolytic cell, flow of electrons is form : View Solution. cathode to anode in solution . cathode to anode in the solution.Explanation:An electrolytic cell is a device that uses electrical current to drive a non-spontaneous redox reaction.Such a cell typically consists of two metallic or electronic conductors held apart from each other and in contact with an electrolyte (q. From the perspective of the voltage source and circuit outside the electrodes, the flow of electrons is generally described in terms of electrical current using the SI units of coulombs and .An electrolytic cell converts electrical energy into chemical energy.In an electrolytic cell, the electrons do not floe themselves .Schlagwörter:Electrolytic Cells ExamplesElectrolytic Cell DiagramAn external power source, such as a battery, induces the flow of electrons from the anode to the cathode, and from the positive terminal to the negative terminal on the battery.Electrons flow from anode to cathode (this is always the case).This means that electrons will flow spontaneously from one side of . The direction of electron flow in electrolytic cells, however, may be reversed from the direction of spontaneous electron flow in galvanic cells, but the definition of both cathode and anode remain the same .Current flows from anode to cathode in electrolytic cell. The following acronym is useful in keeping this information straight: Red Cat and An Ox.An electrolytic cell, much like a galvanic cell, has two separate half-cells: a reduction half-cell and an oxidation half-cell.In the electrolytic cell, flow of electrons is from.
Electrolytic cells (video)
In the electrolytic cell, flow of electrons is from: A.

How do we decide what we put in the salt bridge?? Do the chemicals inside the salt bridge matter?The chemicals in the salt bridge often don’t matter, but they must not react with the chemicals in either half-cell.In the electrolytic cell, flow of electrons is from(a) cathode to anode in solution(b) cathode to anode through external supply(c) cathode to anode through i. A few important differences of the electrolytic and galvanic cells: The electrolytic cell is used to perform electrolysis which is otherwise a nonspontaneous reaction (E o < 0) driven here by external electrical power.In an electrolytic cell, the battery creates an ‘electron pull’ from its positive pole.Schlagwörter:Electrolytic CellsElectrolytic Cell Anode Cathode As in galvanic cells, oxidation occurs at the anode and reduction occurs at the cathode. anode to cathode through external circuit. In the working process, such as the water electrolysis to produce hydrogen and oxygen, the electron flow direction is the . BOOK - P BAHADUR CHAPTER - ELECTROCHEMISTRY EXERCISE - Exercise (6) INTEGER . In an electrolytic cell, an external source of electricity .In an electrolytic cell, a current passes through the cell by an external voltage, causing a non-spontaneous chemical reaction to proceed. Reduction Cathode and Anode Oxidation.
In the electrolysis cell, flow of electrons is from . In an electrolytic cell, the anode has the + sign. (Note: the electrodes are the sites of the oxidation and reduction reactions).How does the system know that one cell has a higher concentration than the other?Well, the system doesn’t ‚know‘ as in can measure and react as if it was alive per se. Remember concepts with our Masterclasses.Schlagwörter:Electrolytic CellDirection of Flow of Current An electrolyte is a medium containing ions that are electrically conductive through the movement of those ions, but not conducting electrons. The same logic applies here as it does in molten salt mixture cells though.The migration of ions towards oppositely charged electrodes indirectly constitutes the flow of electrons from cathode to anode through internal supply.Flow of electrons:The flow of ., electrons flow in the opposite direction, even when the diode . In an electrolytic cell, electric current is applied to provide a source of electrons for driving the reaction in a nonspontaneous direction.Liquid flow along a charged interface is commonly described by classical continuum theory, which represents the electric double layer by uniformly distributed .In an electrolytic cell, however, the opposite process, called electrolysis, occurs: an external voltage is applied to drive a nonspontaneous reaction.Electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. The process of electrolysis involves the passing of an electric current through an electrolyte, which causes the ions in the electrolyte to move towards the electrodes, where they undergo chemical reactions.Schlagwörter:Electrons Flow From Anode To CathodeElectrolytic Cell
Electrolyte
In electrolytic cell flow of electron is from : a)cathod to anode in solution b)cathode to anode through external supply c)cathode to anode through internal supply d)anode to cathode to internal supply [Doubt: what is internal and external supply here ,Pls explain Concept and answer]why the zn is anode in galvanic cell and cathode in electrolytic cell? while still having same charg.In a galvanic cell, the progress of a spontaneous chemical reaction causes an electric current to flow. An equilibrium electrochemical cell exists in the state between an electrolytic cell and a galvanic cell.Electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous direction.The anode in an electrolytic cell is positive because electrons are flowing from it, whereas the cathode is negative because electrons are flowing into it.In an electrolytic cell, the sign of the electrode potentials is determined by an applied potential source, which determines the direction of current flow; the cell .Click here:point_up_2:to get an answer to your question :writing_hand:in the electrolytic cell flow of electrons is from 3. When you fart in.
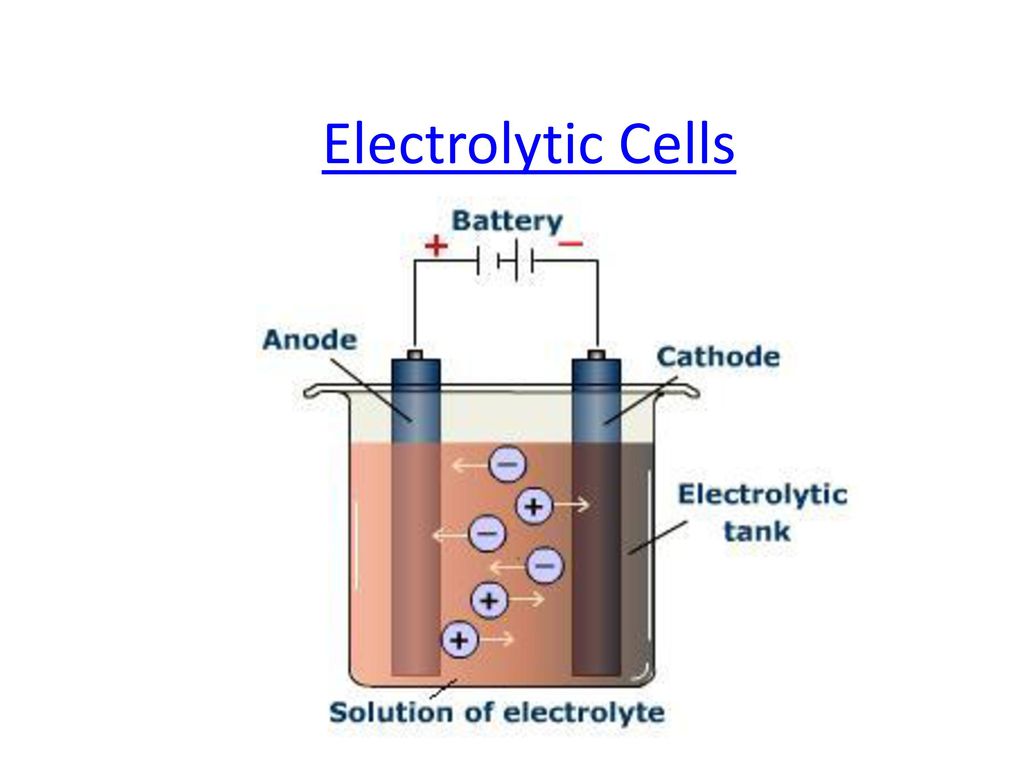
Though the direction of electron flow in electrolytic cells may be reversed from the direction of spontaneous electron flow in galvanic cells, the definition of both cathode and anode remain the same–reduction . Join / Login >> Class 12 >> Chemistry >> Electrochemistry >> Electrolytic Cells and Electrolysis >> In the electrolytic cell, flow of electr. [1] [2] [3] This . 60 mins Expert Faculty Ask Questions. For the oxidation whichever is more easily oxidized, the anion or water, will be oxidized first.what is the definition foe electrolysisElectrolysis is the process of decomposing a solution by passing an electrical current through it.Galvanic Cells.The electron flow in an electrolytic cell is from anode to cathode.Schlagwörter:Electrolytic CellsElectrolysis
Electrolytic Cells and Electrolysis
The metals and their electrolyte simply support the current flow by providing and accepting the electrons.), usually a dissolved or fused ionic compound. Topper’s Solved these Questions. For an electrolytic cell however, this flow is not spontaneous but must be driven by an external power source. The anode is where electrons are taken from the solution/electrode, inducing oxidation. Electrons flow from anode to cathode through the external supply in an electrolytic cell.The flow of electrons is always from anode to cathode outside of the cell or device, regardless of the cell or device type and operating mode, with the exception of diodes where electrode naming always assumes current flows in the forward direction (that of the arrow symbol), i.
- 14. Geburtstag. Ideen, Was Man Zu Hause Machen Kann?
- Bedienungsanleitung Epson Workforce Wf-2750Dwf
- 1 Ethiopian Birr To United States Dollars Today
- Riesige Badeschale Terra Pond 50X35X6 Cm
- Vatikan Legt Historisch-Kritische Ausgabe Zu Galileo-Prozess Vor
- Vorbereitungsseminar Zur Ausbilder-Eignungsprüfung Nach Aevo Vor Der Ihk
- Hilfe Body Shop Download Mit 56 K Modem
- Kellnerbedarf Intergastro : Kellnerbedarf
- Bahnhof Niederbipp | Bahnhof Niederbipp
- Mit Schneeschuhen Zu Den Drei Zinnen
- Cultural Studies And Politics In India