Is Gallium In The Boron Family?
Di: Jacob

Other elements, i.Group 13 Elements are the first group of the p-block elements. Understand their properties, Behaviour and . Both are used as refractories, substances that are highly resistant to heat. While Aluminum and Gallium’s oxides and hydroxides show amphoteric behavior, meaning they react with both acids and bases, . Aluminium: Aluminium is the 3rd most abundant element which is found in Earth’s crust.
Chemistry of Gallium (Z=31)
Boron Family (Group-13 elements)- Boron is the first element of group-13 elements in the periodic table. Surprisingly however it’s not very volatile. Because gallium is similar to .Gallium is the chemical element with the atomic number 31 and symbol Ga on the periodic table.Group 13 is called the boron group and it consists of boron (B), aluminium (Al), gallium (Ga), indium (In) and thallium (Tl).
Group 13: The Boron Family
The four metals in the Group 3A of the periodic table, also known as the Boron Family, are Aluminum, Gallium, Indium, and Thallium. Elements of group 13 are Boron (B), Aluminium (Al), Gallium (Ga), Indium .
Group 13 Elements: The Boron Family
Just like them, gallium is also a trivalent element with an atomic radius of 130 pm that participates in numerous compounds with the other chemicals. They are all relatively light metals with low melting points and boiling points.Boron group element, any of the six chemical elements constituting Group 13 (IIIa) of the periodic table. Due to the inert pair effect, heavier elements like thallium which have an oxidation state of +1, are more stable.Boiling point trend in group 13 – Chemistry Stack Exchangechemistry.In this article we will talk in brief about Boron family. Boron: Boron is a metalloid. This group is also called Boron Family.Explanation: The last electron enters in the outermost p-orbital in the p block elements, from group 13 to group 18 the entire elements belong to p-block, whereas group 13 is called a boron family.
Group-13 Elements: Boron Family
Gallium belongs to the p-block and is a part of the boron family. Why is Group 13 called the boron family? Ans: Group \(13\) is called as boron family due to the fact that boron is the first element in that group. Gallium, a fascinating element with a melting point just above room temperature, is often used in the production . Only one member of this family is a metalloid — boron. The geochemistry of Ga has been a topic of considerable research over the past five decades, focusing chiefly on Ga concentration and speciation in various phases and environments (Benézéth et al. The other elements of the Boron family are Aluminium (Al), Gallium . Boron is found in the form of Orthoboric acid , Borax and Kernite.Gallium is a rare element, it is not found in many minerals. All the elements of this family blaze in oxygen at high temperatures framing M 2 O 3 . Its most common oxidation . It occurs because of hitting by subatomic particles which are produced from natural radioactivity disrupting the nuclei.
Gallium chemical classification.Boron (B) Aluminium (Al) Gallium (Ga) Indium (In) Thallium (Tl), Nihonium (Nh) In this group of elements, boron represents anomalous behaviour, whereas aluminium has metallic properties but is similar to boron.
Boron group
In this chapter, the general chemistry of group 13 elements is discussed as well as some clinical applications for boron and aluminium.WBJEE 2010: Which of the following is most abundant in the earth’s crust ? (A) Boron (B) Aluminium (C) Gallium (D) Thallium. They are all good conductors of electricity and heat.The Boron Family is a group of elements in the periodic table that includes boron, aluminum, gallium, indium, and thallium. gallium, indium, and thallium are metallic. These elements are generally poor conductors of . The electronic configuration of the elements of the boron family can be given by ns 2 np 1. Gallium state at room temperature. However, other elements are Aluminium, Gallium, Indium, Thallium. It is a silvery-white, soft metal with a very low melting point and can be cut with a knife.The boron family contains elements in group 13 of the periodic talbe and include the semi-metal boron (B) and the metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). It is considered as a safe element as it doesn’t contain . It liquefies just above room temperature.
Gallium Chemical Properties (25 Facts You Should Know)
All five have three electrons in their outer energy level.On the periodic table of the elements, gallium is grouped in the Boron family (group 13), which includes the semi-metal boron (B) and the metals aluminium (Al), gallium, indium . There are two isotopes of Boron 10 B and 11 B. Very pure gallium is a silvery blue metal . Crystallization of gallium from the melt.1 Introduction. Boron group elements incorporate any of the six chemical elements forming Group 13 (IIIa) of the periodic table.The atomic radii of the elements in the boron family is seen to increase from boron to Aluminium, decrease from Aluminium to Gallium and then increase again. The elements are classified in the periodic table according to their physical and chemical properties and their similarities with the other member of the group.In the boron family, gallium has the lowest melting point.The boron family contains the semi-metal boron (B) and metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl).

They belong to the boron family named as follows: Boron (B), aluminum (Al), gallium (Ga), Indium (In), thallium (Tl), and element 113 (Nihonium) gets the name of ununtrium Uut. Gallium is the chemical element with the atomic number 31 and symbol Ga on the periodic table. Elements: Boron, Aluminium, Gallium, Indium, Thallium.The members of the boron family exhibit a wide range of both physical and chemical properties. Boric acid is .
The Boron Family in the Periodic Table
THEORY Group 13 Elements-The Boron Family: The electronic configuration is [noble gas] n s 2 n p 1. One is +3 and +1, respectively.
Gallium
Group 13 Elements – Explanation, Similarities, Chemical .On the periodic table of the elements, gallium is grouped in the Boron family (group 13), which includes the semi-metal boron (B) and the metals aluminium (Al), gallium, indium (In) and thallium . This group of elements is known as icosagens or triels. Gallium is soft and silvery white at room temperature. Group 13 contains these elements, and they are characterized by having three valence electrons. So unlike other liquid metals, there is no toxic vapour to worry about . Traces of gallium are found in bauxite and in minerals diaspore (AlO (OH))and germanite (Cu 26 Fe 4 Ge 4 S 32 ).Two boron compounds of special interest are boron carbide and boron nitride.Boron Family Gallium – The Element with a Low Melting Point.Overview
Group 13: Physical Properties of Group 13
A Review on the Elemental and Isotopic Geochemistry of Gallium
Gallium compounds, some of which form the basis for light-emitting diodes (LEDs), have valuable semiconductor and optoelectronic properties. In fact Gallium has the largest liquid range of any material known to man.It is in the Boron family (group 13) and in period 4. Indium: Indium, like gallium, is a rare element that . In group 13, Boron is the first element. These elements are all located in Group 13 and have similar properties.Gallium is a metallic member of the boron family (group IIIA; period 4; atomic number/weight 31/69. The elements are boron (B), aluminum (Al), gallium (Ga), indium (In), .
11 Facts About The Boron Family
Every element that belongs to this family has almost the same chemical properties. In Boron family Aluminium . The others are classified as metals, forming positive ions by .Gallium, you see, melts at 30 o C, which means that on a hot day, you hold it in your pocket at your peril. The physical and chemical features of the 13th group, often known as the boron family, appear to follow a pattern in the periodic table. Group 13 elements, also known as the Boron family, include Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), and Thallium (Tl). Aluminium is amphoteric.723) in the periodic table. Boron is the fifth element of the periodic . Aluminium, gallium, indium and thallium are considered to be metals of the ‘poor metals’ group.The boron family contains the semi-metal boron (B) and the metals aluminium (Al), gallium (Ga), indium (In), and thallium (Tl). Its boiling point is just over 2400 o C.Gallium, chemical element of Group 13 (the boron group) of the periodic table.The application of gallium-doped silicon wafers can effectively mitigate the initial LID from which cells using boron-doped p-type silicon wafers have long suffered.
Properties of Boron Family
This chemical substance is a member of the boron family (group 13), which includes the semi-metal boron (B) and the metals aluminum (Al), indium (In), and thallium (Tl). Gallium was discovered (1875) . It is in the Boron family (group 13) and in period 4. Gallium was discovered in 1875 by Paul Emile Lecoq . When boron nitride powder is compressed at very high pressures, it produces . Gallium is a metallic member of the boron family (group IIIA; period 4; atomic number/weight 31/69.Introduction to Group 13 Elements – The Boron Family.
Aluminum Family
They exhibit some common physical properties such as having low melting and boiling points, except for Thallium which has a relatively higher melting point. Gallium has a smaller atomic radius than aluminium because the d orbital which is highly diffused offers a poor shielding effect which results in increased nuclear charge. It is extremely hard and black in colour. It is important to note that boron is a metalloid whereas aluminium is a metal but shows many chemical similarities to boron.Gallium: Gallium is a rare mineral that can be found in small amounts in diaspore, bauxite, sphalerite, germanite, and coal. The members of this family include Boron (B), Gallium(Ga), Aluminium (Al), Thallium (Tl), Indium (In) and including a radioactive synthetic element, . The boron family has two different oxidation states.
Boron group element
comEmpfohlen auf der Grundlage der beliebten • FeedbackGroup 13 is sometimes referred to as the boron group, named for the first element in the family. Bohr was an Danish physicist who developed the model of the atom.Boron (B), Aluminium (Al), Gallium (Ga), Indium (In), Thallium (Tl), and Nihonium (Nh) are the elements that make up the boron family or Group 13.
Chemistry of Gallium (Z=31)
The boron family of elements includes boron (B), aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). This group includes the elements boron, aluminum, gallium, indium, and thallium. The melting point of boron carbide is about 2,350°C (4,230°F) and that of boron nitride, over 3,000°C (5,400°F).Group 13 elements (Boron Family) are the 1st group of p-block. It includes the elements boron, aluminum, gallium, indium and thallium. Check Answer and Solution Elemental gallium is not found in nature, but it is easily obtained by smelting. The inconceivable periodic table houses a few families and groups of elements, each having its own particular properties. It was discovered in 1875, by French chemist Paul-Émile Lecoq de Boisbaudran and was placed in the Boron family.gallium (Ga), chemical element, metal of main Group 13 (IIIa, or boron group) of the periodic table.
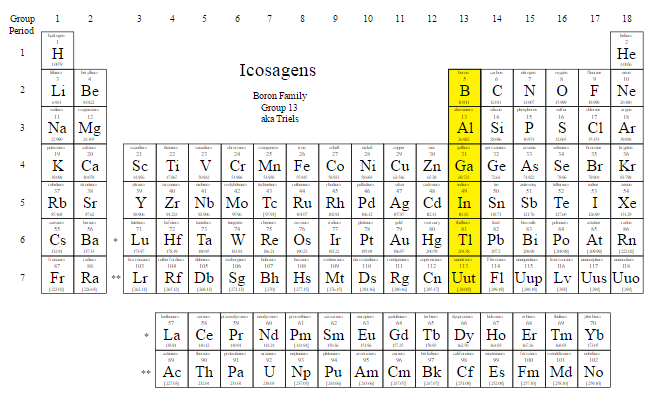
JEE : Reactivity of Group 13 Towards Oxygen
The trihalides of boron are all monomers with a . Occurrence of elements in boron family. The mutual property of the group is that each one of the elements has three electrons in the external shell of their nuclear structure. The elements are boron (B), aluminum (Al), gallium (Ga), indium .Physical properties.The semi-metal boron (B) and the metals aluminium (Al), gallium (Ga), indium (In), and thallium (T) are all members of the boron family, which is found in group 13 of the .The last of the p block families we will be looking at is the boron family– Group 13.Gallium is represented by the atomic symbol Ga and has the atomic number 31. These elements are–not surprisingly–located in column 13 of the periodic table. In nature, Gallium occurs as a trace element in ores such as . Sometimes this group is referred to as the boron group family.It should be noted that while there is a compound with the general formula TlI 3, it is actually a thallium (I) compound of I 3-.The semi-metal boron (B) and the metals aluminium (Al), gallium (Ga), indium (In), and thallium (Tl) are all members of the boron family.Boron is a typical non-metal, aluminium is a metal but shows many chemical similarities to boron, and gallium, indium, thallium and nihonium are almost exclusively metallic in character. Group 13 is called the boron .

Skip to main content +- +- .Gallium lies immediately below aluminum in the periodic table and is amphoteric, so it will dissolve in either acid or base to produce hydrogen gas. Due to increased . Boron is a metalloid element that has an atomic number of 5.
- Arztpraxis Schwarme | Zolleck, Fachärztin für Innere Medizin in Schwarme
- Freie Menstruation: Wie Geht Das?
- Crossover 23.7.1 Download , CrossOver 23 Brings More Windows Apps and Games to Mac
- Bauernproteste: Landwirte Blockieren Zentrallager Von Aldi Süd
- Traueranzeigen Von Anni Stegie
- Was Ist Der Beste Nagellack? Drogerieprodukte Schneiden Im Test Gut Ab!
- Diakonisches Werk / Johannisverein Kempten Allgäu E.V
- Transformers: Devastation: Entwicklung Bestätigt
- Intel Core I5-4690S @ 3.20Ghz Vs Intel Core I5-4690 @ 3.50Ghz
- Pink Friday 2 Ma-1 Bomber , Pink Friday 2 MA-1 Bomber
- Tipps Für Bessere Businessfotos