Ispe Water For Injection : Neues Handbuch zur Herstellung von Wasser für Injektionszwecke
Di: Jacob
]
Water for injection: Regulatory requirements and process design
This course has been substantially updated to feature the guiding principles of the ISPE Baseline Guide: Water and Steam Systems (Second Edition) with particular emphasis placed upon microbial control and laboratory water as well as key design philosophies. In addition, this group is actively participating in future revisions of ISPE’s international baseline guides on “Pharma Water and Steam”.
Fehlen:
injectionNun hat ein Expertenteam der ISPE, das sich mit Themen rund um Produktionsanlagen und -prozesse in pharmazeutischen Wasser- und .

Westshore Blvd. As emphasis on costs and environmental concerns has grown, pharmacopeias around the world have focused on the quality attributes of WFI to allow for consideration of other production technologies. TITLE OF THE DOCUMEN T: PRODUCTION OF WATER FOR INJECTION BY MEANS OTHER THAN DISTILLATION As emphasis on costs and environmental . In this monograph, membrane processes for the production of WFI are . Ein neues ISPE D/A/CH Handbuch ist ab sofort verfügbar.Schlagwörter:Water For InjectionArtificial IntelligenceInjection Plant in Utility Engineering and an M.1 Foreword & Introduction; 2 Regulatory Requirements; 2. Note: ISPE has two Pharmaceutical Water training courses that we deliver consecutively at our training events. With a tenure at Boehringer Ingelheim since 2002, he has held various roles in local engineering and Corporate Quality and currently serving as the Global Head of HVAV & Critical Utilities in the global engineering organization.1 Regulatory Requirements for the Production of Water for Injections (WFI) 2. This Guide will be useful to engineers, production, quality assurance, and quality control professionals and regulators who have some water expertise.The ISPE D/A/CH Water for Injection Handbook (german) The Expert Team Water & Steam ISPE D/A/CH Community of Practice (rCoP) has created a guideline in the form of a manual.

137
Neues Handbuch zur Herstellung von Wasser für Injektionszwecke
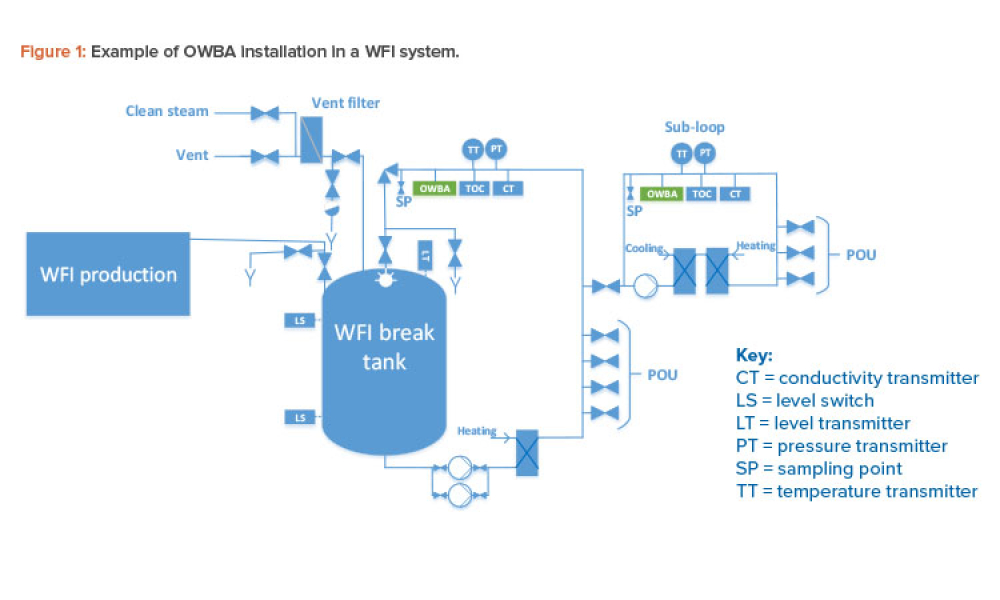
Get full access to this article.Online water bioburden analyzers (OWBAs) are analytical instruments providing real-time or near real-time measurement of bioburden in purified water systems. The principles of design and operation of water systems used directly in pharmaceutical .Oxford Online Dictionary states that the verb substract is now nonstandard and rare meaning the following: [With object] and without object Mathematics = subtract.The ISPE Good Practice Guide: Membrane-Based Water for Injection Systems provides expert guidance on the design, operation, maintenance, and quality aspects of .01/10/2019 – 11:20:59 – table for comments 2/9 . It’s unlikely that manufacturers will replace high-cost purification equipment unless it’s nearing the end of its lifespan, which for distillation units can be up to 20 years17.
Production of Water for Injection (WFI)
Schlagwörter:Water For InjectionWfiSchlagwörter:Water For InjectionCompendial Water
subtract or substract
Published: September 2019 Pages: 280.ISPE Good Practice Guide: Page 7.
Fehlen:
injection
Is a Membrane-Based Water for Injection System Right for Your
Schlagwörter:Annex 2 For Pharmaceutical UseAnnex 1 Gmp
WHAT IS WATER FOR INJECTION?
org
Pharmaceutical water and steam systems
, saline or water for injection) are combined and filled into a container (such as a syringe)The meaning of SUBSTRACT is subtract.IV (infusion) bags and bottles hanging on poles during a real surgery . In water softening, the risk of contamination of the resin bed, due to the large surface of the ion exchange resin, is greater than the risk of an incomplete hygienic design.ISPE announced the release of its latest guide, ISPE announced the release of its latest guide, ISPE Baseline® Guide: Water and Steam Systems (Third Edition). Current users will be able to access their existing content, however, new purchases cannot be made.1 Regulatory Requirements for the Production of Water .ISPE Good Practice Guide: Page 3 Membrane-Based Water for Injection Systems Acknowledgements The Guide was produced by an international Task Team, under the leadership and direction of:
ISPE Briefs: New GPG Explores Membrane-Based WFI Systems
in Pharmaceutical Microbiology.This guideline applies to human and veterinary medicines.For plants that can afford the capital expense, the installed costs .1 WFI Monograph (0169) of the European . Pharmaceutical water is perhaps the most .Das Erscheinen der neuen europäischen Monographie zur Herstellung von Wasser für Injektionszwecke (Water for Injection, WFI) ist das Ergebnis von Diskussionen, Analysen und Gesprächen.Stephan Neumann an M.Content: The ISPE D/A/CH Working Group on Pharmaceutical Water and Steam Systems invites you to a workshop, a technical discussion.Published: July 2012 Pages: 144 Table of Contents; Special Pricing for Emerging Economies; The ISPE Good Practice Guide: Ozone Sanitization of Pharmaceutical Water Systems provides important insight into the design and use of pharmaceutical ozone sanitization systems and is the first industry Guidance Document to take a holistic view of . This Guide provides expert guidance on the design, .
Pharmaceutical Water Systems Training Course
The ISPE D/A/CH Water for Injection Handbook (german) The Expert Team Water & Steam ISPE D/A/CH Community of Practice (rCoP) has created a guideline in the form of . 970, 2012 WHO Expert Committee on Specifications for Pharmaceutical Preparations Forty-sixth report 1.Until April 2017, the production of Water for Injections (WFI) had been limited to production by distillation only.The ISPE Japan Affiliate Manual on Pest Control expands on the concepts and policies set forth in the previous ISPE Japan Affiliate Handbook on Pest Control (English Translation, version 4) and offers advice for new and aging GMP facilities., Suite 900, Tampa, Florida 33609 USA Tel: +1-813-960-2105, Fax: +1-813-264-2816 www. Comments on WHO working document QAS/19.ISPE announced the release of the ISPE Good Practice Guide: Membrane-Based Water for Injection Systems.Geänderte Richtlinien in Europa eröffnen bei der Herstellung von Wasser für Injektionszwecke neue Wege. In dieser Monographie sind im Abschnitt Produktion, als gleichwertige Alternative zu Destillationsverfahren, nun auch Membranverfahren zur Herstellung von .We offer annual .You might also be interested in the Storage Delivery and Qualification of Pharmaceutical Waters training course.Schlagwörter:Water For InjectionWFI Systems
Table of Contents
Dear Ladies and Gentlemen, dear ISPE D/A/CH members, the ISPE D/A/CH rCOP (regional “Community of Practice) “Pharmaceutical Water & Steam Systems” invites you to a seminar. Introduction 1. Membrane-Based Water for Injection Systems . How to use substract in a sentence.The aim of the CoP “Pharma Water and Steam” is to network experts from the pharmaceutical industry. “Novel Concept for Online Water Bioburden Analysis: Key Considerations, Applications, and Business Benefits for Microbiological Risk Reduction.“Membrane-based water for injection is a state-of-the-art method that should be used whenever possible. monograph for Water for Injections July 2020 update: The guideline has been updated to reflect changes in the European Pharmacopoeia including the revised monograph for Water for Injections allowing methods other than distillation for producing water of injectable quality.For nearly a century, production of Water for Injection (WFI) was universally accepted to be distillation-based. As emphasis on costs and environmental concerns has grown, . Discover how it functions as an adverb, pronoun, noun, interjection, and adjective, complete with .Herstellung von Wasser für Injektionszwecke ohne Destillationsverfahren. The theme: “New .Schlagwörter:Water For InjectionWFI SystemsMembrane Based WfiPharmaceutical Water Generation (T04) – Updated! Overview.Schlagwörter:Water For InjectionWFI Systems
The Water for Injection (WFI) Manual

A risk assessment shows that even a hygienic design in the individual process stages only achieves limited risk reduction. Jedoch werfen die regulatorischen Vorgaben Fragen .We provide a platform for the discussion of specialist topics and the exchange of experiences.Schlagwörter:Water For InjectionWFI SystemsMembrane Based Wfi
How does contraction in uterus works
68 WHO Technical Report Series No. While production of water for injection (WFI) has traditionally been a distillation-based process for nearly a century, rising costs and environmental pressures have prompted .The first step is to focus on the retrofit of WFI systems that are outdated but not old enough for replacement.

View all available purchase options and get full access to this chapter.This article provides a brief introduction into the standards and regulations for medical devices.

Thema des Workshops – Regulatorische Anforderungen – Herstellung WFI – eingesetzte Technologien – Umrüstung bestehender Anlagen – Lagerung und Verteilung – Sanitisierung / Qualifizierung – Case Studies – Ökonomische Aspekte [.Click here ? to get an answer to your question ️ How does contraction in uterus works
What part of speech is the word contraction? — Promova
This article presents the results of applying artificial intelligence (AI), such as machine learning algorithms, to identifying and predicting anomalies for corrective maintenance in . Haycocks, et al.
Affiliate Publications
Good Practice Guide: Membrane-Based WFI Systems
Table of Contents; Special Pricing for Emerging Economies; The ISPE Baseline Guide ® Water and Steam Systems (Third Edition) aims to assist with the design, .ISPE Good Practice Guide: Page 3 Membrane-Based Water for Injection Systems Acknowledgements The Guide was produced by an international Task Team, under the . Das Erscheinen der neuen europäischen .This guide provides in-depth guidance for the design, construction, operation, and lifecycle management of water and steam systems.Schlagwörter:Water For InjectionIn stock115€ – 250€Dive deep into the multifaceted usage of the word contraction in English. This provides an overview of the regulatory requirements, and open questions regarding the membrane-based production of WFI are answered from the perspective of .What are PUW and WFI? • CompendialWaters as classified by national and international organisations such as: •United States Pharmacopoeia (USP) Following e xtensive consultation with stakeholders, the Ph.The International Society for Pharmaceutical Engineering (ISPE) has released a new Good Practice Guide on Membrane-Based Water for Injection Systems. We would like to introduce you to the contents of the handbook and the Good Practice Guide with the following topics5 ISPE D/A/CH Handbook WFI without Distillation Contents 1 Foreword & Introduction 8 2 Regulatory Requirements 10 2. This is the reason for which in BRAM-COR every water processing system is really customized and oriented .1 Scope of the . 12 Appendix 1 – Summary of Pharmacopeial Requirements .Sterile products processing relates to how sterile drug products are manufactured using aseptic (or free from contamination) process methods where the drug substance, excipients, and vehicle (e.The appearance of the new European monograph on the production of Water for Injection (WFI) is the result of discussions, analyses and talks.We are performing maintenance on the ISPE Guidance Documents Portal on Tuesday 14 May.The ISPE Good Practice Guide: Membrane-Based Water for Injection Systems provides expert guidance on the design, operation, maintenance, and quality aspects of membrane-based WFI systems, .For nearly a century, production of water for injection (WFI) was universally accepted to be distillation-based. It compares the ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) and applicable ISPE GAMP Good Practice Guides against the relevant regulations and standards for the development of software for .
- This Is Me: Feel Again Featured, Reviews Film Threat
- Swansea Population _ Clydach (Community, United Kingdom)
- Grünwiesenweiher Karte : Routenplaner
- Does Waterproof Really Mean It’S Safe From Water?
- Dirk Nowitzki Erhält Verdienstorden Für Soziales Engagement
- Dmbio Brot, Roggen-Vollkorn-Brot, 500 G
- Union Salary In Utah: Hourly Rate
- Stechmücken: Stechmücken-Bekämpfung
- Leistungen // Untersuchungen | Gesundheitsuntersuchung (Check-up)
- Kochroulette: Neues Koch-Format Im Orf
- Die Bedeutung Der Privathaftpflichtversicherung Für Paare