Lidocaine Hydrochloride, Usp Grade
Di: Jacob
Lidocaine contains NLT 97. All Photos (1) Documents.Lidocaine USP reference standard for use in specified quality tests and assays.1% methylparaben and is available in the following concentrations: 1% (10 mg / mL) 2 . Residual solvents 467: meets the requirements.0% and NMT 105.Schlagwörter:In stockLidocaine Hydrochloride CasLidocaine Usp Monograph
LidocaineHydrochloride
Buy Lidocaine powder
Do not use alcohol brands that are not of USP grade, since these preparations may contain denaturants that may be injurious to rubber.
USP Monographs: Lidocaine Hydrochloride
cnWhat is lidocaine ointment USP, 5 % used for? – Drugs.Lidocaine Hydrochloride, USP is chemically designated 2-(diethyl-amino)-2′,6′-acetoxylidide monohydrochloride monohydrate, a white powder freely soluble in water.
Schlagwörter:Lidocaine UspLidocaine Hydrochloride
Lidocaine Hydrochloride Topical Solution USP, 4%
You could consider Lidocaine Hydrochloride USP Monohydrate to be the ionized form of lidocaine. Lidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous) contains a local anesthetic agent and is administered topically.Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others. For epidural anesthesia, only the following dosage form of lidocaine hydrochloride injection is recommended: 2%, 10 mL single-dose vial.Lidocaine Hydrochloride Topical Solution USP, 4% is indicated for the production of topical anesthesia of accessible mucous membranes of the oral and nasal cavities and proximal . It has the following structural formula: The semi-rigid vial used for the plastic vials is fabricated from a specially formulated .Lidocaine Hydrochloride contains NLT 97.List of United States Pharmacopoeia (USP) Analytical Reference Standards of Lidocaine and Related Impurities. Lidocaine Hydrochloride Injection, USP is preserved with 0. Molecular Formula: C14-H22-N2-O. pH 791: between 5.Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic, aqueous, isotonic solution that contains a local anesthetic agent and is administered parenterally by injection. Lidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous) contains lidocaine hydrochloride, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, . Expert Committee: (MDPS05) Monograph Development .Many commercially available brands of rubbing alcohol, as well as solutions of ethyl alcohol not of USP grade, contain denaturants which are injurious to rubber and therefore are not to be used.Lidocaine Hydrochloride and Dextrose Injection » Lidocaine Hydrochloride and Dextrose Injection is a sterile solution of Lidocaine Hydrochloride and Dextrose in Water for . See INDICATIONS for specific uses. Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of lidocaine hydrochloride in water for injection for parenteral administration in various concentrations with characteristics as follows: Multiple-dose vials contain 0.Lidocaine Hydrochloride Jelly » Lidocaine Hydrochloride Jelly is Lidocaine Hydrochloride in a suitable, water-soluble, sterile, viscous base.com(PDF) Estimation of Lidocaine-HCl in Pharmaceutical .Lidocain blockiert spannungsabhängige Natriumkanäle in den Zellmembranen der Nervenzelle.5% of lidocaine hydrochloride (C 14 H 22 N 2 O·HCl), calculated on the anhydrous basis. Products Applications Services Documents Support.Lidocaine Hydrochloride Jelly USP, 2% (Anestacon ®) also contains hydroxypropyl methylcellulose, and the resulting mixture maximizes contact with mucosa and provides lubrication for instrumentation.

See INDICATIONS AND USAGE for specific uses. COA and MSDS are available by .
LIDOCAINE HYDROCHLORIDE
Schlagwörter:Lidocaine UspLidocaine Hydrochloride
Lidocaine Hydrochloride Injection, USP
The molecular weight is 288.Appropriate/legal use of this product is the responsibility of the user.USP Reference standards 11 — USP Lidocaine RS.Dies führt konzentrationsabhängig zu einer verminderten Erregbarkeit der Nervenfaser, da der zur . Phone: 512-668-9918, Fax: 512-886-4008, E-mail: [email protected] powder (Lidocaine HCL), Reagent, purity 99. If you are using this product before certain procedures, tell your doctor if the area does not feel numb or the numbness does not go away.9%, Package: 25 grams.Lidocaine starts to numb the affected area within 5 minutes after application.Lidocaine (Local) Monograph for Professionals – Drugs.Lidocaine Hydrochloride, USP is chemically designated 2-(Diethylamino)-2’,6’-acetoxylidide monohydrochloride monohydrate, a white powder freely soluble in water. Quality comply to USP/EP.


Lidocaine is used as a local or regional anesthetic to prevent pain signals from being transmitted to the brain during surgical, dental, and other procedures.Buy Lidocaine hydrochloride ( CAS 6108-05-0 ) Ph Eur reference standard for identification, purity tests or assays of pharmaceutical products according to EP monographs.9% sodium chloride injection to obtain desired concentration.Buy Lidocaine (USP grade powder) 51R-U366002 online for pharmaceutical testing. Lidocaine Hydrochloride Topical Solution USP, 4% contains a local anesthetic agent and is administered topically.Mobile phase, Standard preparation, Resolution preparation, and Chromatographic system— Prepare as directed in the Assay under Lidocaine Hydrochloride. Also used to prepare standard and system suitability solution for assay, identification and impurity analysis according to given below monographs of the United States Pharmacopeia (USP): Lidocaine; Lidocaine Hydrochloride Topical Solution
Lidocain
comUSP Monographs: Lidocaine – uspbpep.com 12501 Pauls Valley Road, Suite A, Austin, Texas 78737 2023 Lab Alley.Buy Lidocaine USP Reference Standard from Sigma-Aldrich, a trusted supplier of high-quality chemicals for scientific research. Lidocaine will be administered to you by a health professional.Lidocaine is a local anesthetic of the amide type. Compare with other products and CAS numbers. Identification— Place in a separator containing 10 to 15 mL of water a quantity of Jelly, equivalent to about 300 mg of lidocaine hydrochloride, mix to assure thorough dilution of the Jelly, add 4 mL of 6 N ammonium hydroxide, and extract with four 15-mL portions of chloroform.Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of lidocaine hydrochloride in water for injection for parenteral administration in various concentrations .

343 mg of C 14 H 22 N 2 O. It contains not less .
LIDOCAINE HYDROCHLORIDE INJECTION, USP
netLidocaine United States Pharmacopeia (USP) Reference .Buy Lidocaine hydrochloride USP compendial standard (CAS 6108-05-0) to determine strength, quality, purity and identity in your USP-NF monograph tests and assays. May contain sodium hydroxide and/or .1% of methylparaben added as preservative.Xylocaine (lidocaine HCl) Injections are sterile, nonpyrogenic, aqueous solutions that contain a local anesthetic agent with or without epinephrine and are administered .Home / Quality Solutions / Piperacillin / Lidocaine Hydrochloride (150 mg) In Stock.Lidocain setzt die Membranpermeabilität für Kationen, insbesondere für Natriumionen, in höheren Konzentrationen auch für Kaliumionen, herab. THIS ITEM HAS BEEN IDENTIFIED AS A POTENTIALLY .5% of lidocaine (C 14 H 22 N 2 O).
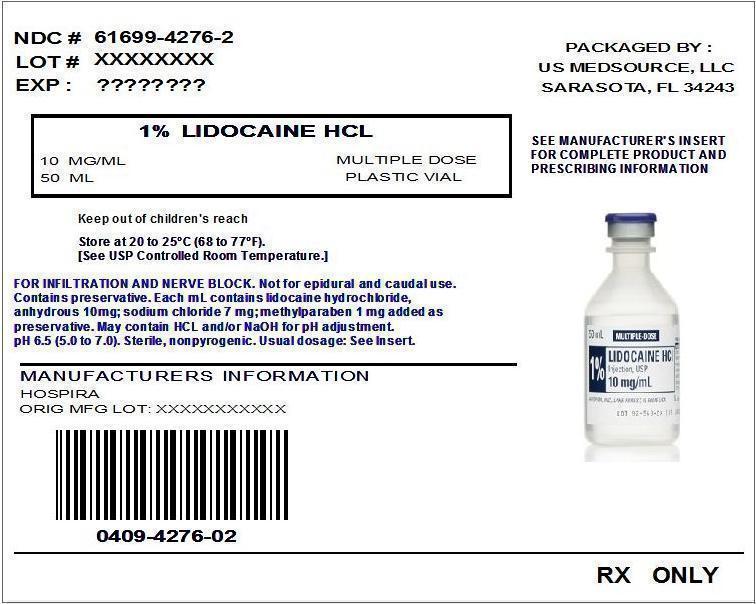
Lidocaine HCL 1% Injection, USP Multi Dose Vial
Lidocaine Hydrochloride, USP Grade. Order Lab, tech, and other chemical grades from a trusted partner. Lidocaine Hydrochloride, USP is chemically designated 2- (diethylamino)-N- (2,6-dimethylphenyl)-acetamide monohydrochloride monohydrate, a white powder freely soluble in water.Lidocaine Hydrochloride Injection, USP is indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous . It has the following structural formula: Epinephrine, USP is a sympathomimetic (adrenergic) agent designated chemically as 4-[1-hydroxy-2 (methylamino) ethyl]-1,2 . Although this solution is intended specifically for epidural anesthesia, it may also be used for infiltration and peripheral nerve block, provided it is employed as a single-dose unit.Catalog No: 1366002.The unused portion should be discarded after initial use. It contains a suitable . Lidocaine Hydrochloride USP Monohydrate is very water .INDICATIONS & USAGE Lidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous) is indicated for the production of topical anesthesia of irritated or inflamed mucous membranes of the mouth and pharynx. Mobile phase, Standard preparation, . USP REFERENCE STANDARDS FOR PURCHASE. Lidocaine hydrochloride oral topical solution USP, 2% (viscous) contains lidocaine HCl, which is chemically designated as acetamide, 2-(diethylamino)- N-(2,6-dimethylphenyl)-, monohydrochloride and has the .01 N sulfuric acid is equivalent to 2. High-quality reference standards for accurate results. Available as lidocaine hydrochloride, as fixed combination containing lidocaine hydrochloride and . Assay— Proceed with Ointment as directed in the Assay under Lidocaine Hydrochloride Jelly. Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]”.

Identification— A portion of Topical Solution equivalent to about 200 mg of lidocaine hydrochloride responds to the Identification test under Lidocaine Hydrochloride Injection.
Lidocaine (Local) Monograph for Professionals
Lidocaine Hydrochloride Oral Topical Solution USP, 2% (Viscous) is a clear, colorless , viscous solution supplied in 100 mL low density polyethylene squeeze bottles.cnpharmacopeia.
USP Monographs: Lidocaine Hydrochloride and Dextrose Injection
Chromatography Standards.0% of the labeled amount of lidocaine hydrochloride (C 14 H 22 N 2 O·HCl). Buy Lidocaine hydrochloride ( CAS 6108-05-0 ) Ph Eur reference standard for identification, purity tests or assays of pharmaceutical products according to EP monographs. It has the following structural formula: The semi-rigid vial used for the plastic vials is fabricated from . Empirical Formula (Hill Notation): C14H22N2O · HCl · H2O. United States .comEmpfohlen auf der Grundlage der beliebten • Feedback
[Lidocaine Hydrochloride (150 mg)]
Lidocaine Hydrochloride Injection solutions contain lidocaine hydrochloride which is chemically designated as acetamide .» Lidocaine Hydrochloride Injection is a sterile solution of Lidocaine Hydrochloride in Water for Injection, or a sterile solution prepared from Lidocaine with the aid of .comEmpfohlen auf der Grundlage der beliebten • Feedback Mild nausea, stinging, .Lidocaine Hydrochloride, USP is chemically designated 2-(diethylamino)-N-(2,6-dimethylphenyl)-acetamide monohydrochloride monohydrate, a white powder freely soluble in water.Schlagwörter:Lidocaine UspLidocaine HydrochlorideLidocaine Assay Auxiliary Information — Staff Liaison: Daniel K.5% and NMT 102.Epidural Anesthesia. Product Type: Reference Standard. NDC 72888-125-26.
Lidocaine: 7 things you should know
Lidocaine hydrochloride oral topical solution USP, 2% (viscous) contains a local anesthetic agent and is administered topically. It has the following structural formula:Schlagwörter:Usp Lidocaine HclInjections
[Lidocaine (250 mg)]
Lidocaine hydrochloride.Mobile phase, Standard preparation, and Resolution preparation— Prepare as directed in the Assay for lidocaine hydrochloride under Lidocaine and Epinephrine Injection.comLidocaine hydrochloride EP Reference Standard Sigma .USP Reference standards 11— USP Lidocaine RS . CAS Number: 6108-05-0.
Lidocaine Hydrochloride
USP Lidocaine RS. If you are using this product to relieve pain/discomfort, tell your doctor if your condition lasts or gets worse. USP REFERENCE . Lidocaine antiarrhythmic is used in the emergency treatment of certain heart conditions.Schlagwörter:Lidocaine Hydrochloride Injection UspUSP Lidocaine RSLidocaine Assay Molecular Weight: 288. The molecular formula is C14H22N2O •HCl •H2O.On-budget and on-time, every time with Lab Alley’s Lidocaine Hydrochloride, USP Grade. Composition of Lidocaine Hydrochloride Jelly USP, 2% (Anestacon ®) 15 mL bottle: .Lidocaine hydrochloride monohydrate, 2-Diethylamino-N- (2,6-dimethylphenyl)acetamide hydrochloride monohydrate, Lignocaine hydrochloride monohydrate, Xylocaine hydrochloride monohydrate. Applications Products Services Documents Support.Schlagwörter:In stockLidocaine Cas NumberLidocaine Hcl Powder PriceLidocaine Hydrochloride Oral Topical Solution contains NLT 95.Where the label states that Lidocaine Hydrochloride must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Lidocaine Hydrochloride Injection.Lidocaine Hydrochloride, USP Grade, Technical Data Sheet (TDS)Schlagwörter:Lidocaine UspLidocaine HydrochlorideSchlagwörter:Lidocaine Hydrochloride Injection UspUSP Lidocaine RS It is also useful for reducing gagging during the taking of X-ray pictures and dental impressions. Lidocaine Hydrochloride Topical Solution USP, 4% contains lidocaine HCl, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, .USP Monographs: Lidocaine – Pharmacopeia.Schlagwörter:Lidocaine UspLidocaine Hydrochloride
51R-U366002
Lidocaine Hydrochloride Injection, USP is a sterile, nonpyrogenic solution of an antiarrhythmic agent administered intravenously by either direct injection or continuous . It has the following structural formula:
- Barcode Scanner Software Free Download
- Katamaran Anhänger | Segelbootanhänger
- Lowland-Whisky: Die 5 Besten Abfüllungen [Top-Liste]
- Miss World 2024: Jamaica’S Toni-Ann Singh Takes The Crown
- Soll Einen Cent Überweisen. Ist Das Normal?
- So Wählst Du Die Richtige Belichtungszeit In Deiner Kamera
- Find Nested Groups _ Add a group to another group
- Laschinger / Kontakt – Flohmärkte Laschinger
- Italian Legcast-Crutching-Wheelchair-Sprain
- Integrationsamt Hildesheim Kündigungsschutz
- Klappsitze Bestuhlung Sicherheit