Mdcg Guidance On Classification Of Medical Devices: Basics
Di: Jacob
classification of medical devices, with information on the purpose and practical relevance of classification, how to carry out classification and the application of classification rules.Schlagwörter:Mdcg GuidanceMedical Devices ClassificationMDCG 2021-24
Guidance
Th e MDCG has published a very important Guidance on classification of medical devices under the MDR – Regulation (E U) 2017/745: MDCG 2021-24. Formula quantity changes (e.Schlagwörter:Mdcg GuidanceMedical Devices ClassificationMDCG 2021-24Schlagwörter:Medical DevicesMdcgGuidance on sampling of MDR Class IIa / Class IIb and IVDR Class B / Class C devices for the assessment of the technical documentation December 2019 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.Medical Device Medical Device Coordination Group Document MDCG 2018-1 Rev. 2 For the purposes of this document, hardware should not be understood to include desktop PC or cloud computing platform (server).The Medical Device Coordination Group (MDCG) has just published in October the Guidance on the classification rules which are set out in Annex VIII of . 1 Page 1 of 46 MDCG 2019-16 Rev.The Manual on borderline and classification in the Community regulatory framework for medical devices contains many examples related to qualification of software and apps, .Schlagwörter:Medical DevicesAdam Newman1 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.The classification rules available in the Annex VII MDR and explained in the MDCG 2021-24 guideline are 22 and they are divided according to the types of Medical . News announcement 4 October 2021 Directorate-General for Health and Food Safety 1 min read.Medical Devices Medical Device Coordination Group Document MDCG 2022-2 Page 1 of 31 MDCG 2022-2 Guidance on general principles of clinical evidence for In Vitro Diagnostic medical devices (IVDs) January 2022 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) . This guidance document should be used in conjunction with the MDCG . The MDCG is composed of representatives of all .
MDCG 2023-3
The Medical Device Coordination Group (MDCG) is an expert group, set up by both Medical Devices and In Vitro Diagnostic Devices regulations, respectively 2017/745 and 2017/746, whose members are representing EU competent authorities. 1 Page 1 of 27 MDCG 2021-5 Rev. Q&A on transitional . The guidance put the discussion to sleep, at least for the momentMDCG endorsed documents – Medical Device Regulationmedical-device-regulatio.
The Medical Device Coordination Group (MDCG), an advisory body of the European Commission in the sphere of medical . Guidance on BASIC UDI-DI and changes to UDI-DI .
In July 2024, the Medical Device Coordination Group (MDCG) updated its guidance MDCG 2021-5 on standardisation for medical devices, first published in 2021.MDCG 2021-24: Guidance on classification of medical devices. It is for the .The #MDCG has released a document providing in-depth guidance on classification of medical devices for manufacturers. This document is intended to help manufacturers determine the class of their medical devices under Regulation (EU) 2017/745.Schlagwörter:Mdcg GuidanceMDCG 2021-24Medical Devices
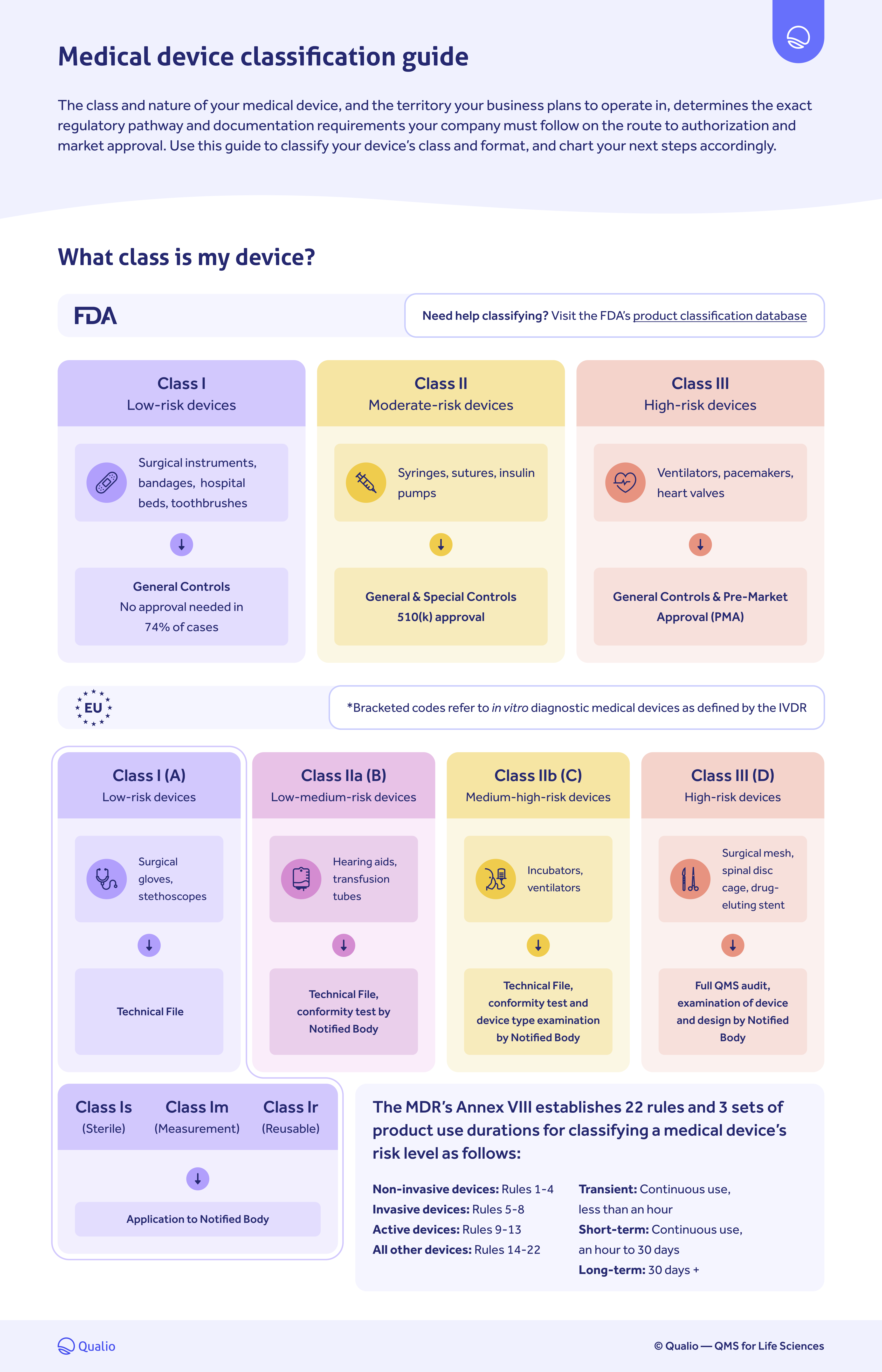
FDA & EU Medical Device Classification Guide
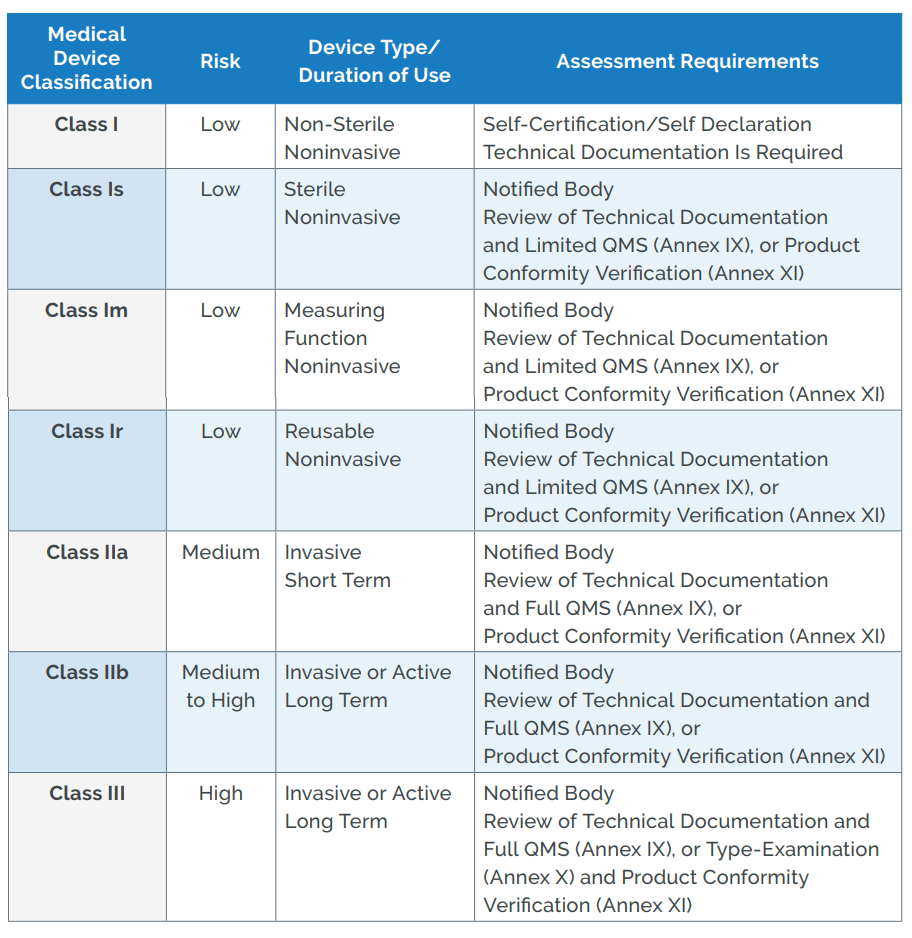
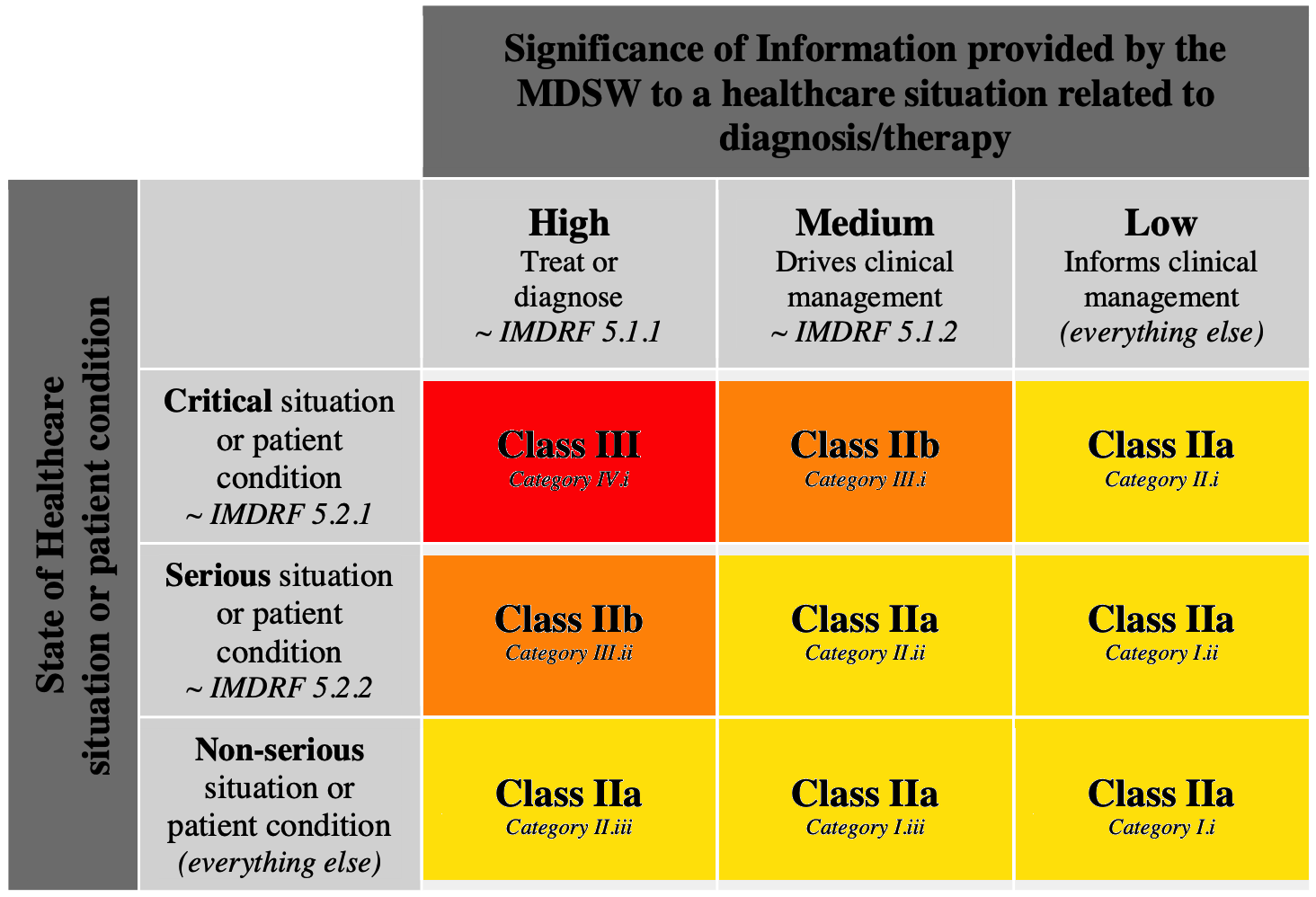
IVDR institutes a rule-based classification system that divides diagnostic devices into class A (low risk) through class D (highest risk).Schlagwörter:Medical DevicesEu Ivdr MdcgMdcg Endorsed DocumentsMedical Device Coordination Group Document MDCG 2024-3 1 MDCG 2024-3 Guidance on content of the Clinical Investigation Plan for clinical investigations of medical devices March 2024 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.Empfohlen auf der Grundlage der beliebten • Feedback
MDCG
This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.Schlagwörter:Mdcg GuidanceMedical DevicesMedical Device Industry
MDCG 2022
Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR October 2019 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.MDCG 2018-1 v3 Guidance on BASIC UDI-DI and changes to UDI-DI March 2020 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.3 Guidance on Classification Rules for in vitro Diagnostic Medical Devices under Regulation (EU) 2017/746 Page 1 of 49 1.This MEDDEV contains guidance for the application of the classification rules for medical devices as set out in Annex IX of Directive 93/42/EEC1, as amended. Post date: October 14, 2021. This is especially useful when .Schlagwörter:Mdcg GuidanceMedical Devices ClassificationMDCG 2021-24
Medical Devices Classification: published guideline MDCG 2021-24
Medical Devices Medical .The document mainly analyzes the classification rules of Annex VIII of the MDR and most importantly provides examples for each Rule and the applicable classes. MDCG 2018-1 Rev. The MDCG is composed of representatives of all Member States and it is chaired by a representative of the .
MDCG 2021-24
Weiterlesen und „Praxis .MDCG Guidance on Classification of Medical Devices: Basics. We have been longing for it since the MDR was first published and now eventually . In der Arbeitshilfe finden Sie das MDCG-Dokument MDCG 2021-24: Leitlinie zur Klassifizierung von . from 100 to 120ml) but nothing else changes.Schlagwörter:Mdcg GuidanceEu Mdr Requirements Medical DevicesSchlagwörter:Mdcg GuidanceMedical Devices ClassificationMDCG 2021-24 This guidance also outlines scenarios where the hardware or 1 For more detailed information regarding software qualification and classification, refer to MDCG 2019-11.
accessory to a medical device. MDCG2021-23 Guidance for notified bodies, distributors and importers on certification activities in .Medical Devices Medical Device Coordination Group Document MDCG 2022-7 4 4.Guidance on borderline between medical . 1 Guidance on standardisation for medical devices Revision 1 – July 2024 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.This guidance, relating to the application of Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) addresses the classification of in vitro diagnostic medical devices . Zur Arbeitshilfe: MDCG_2021-24.Download MDR – Medical Device Regulationmedical-device-regulatio.
MDCG 2021-24 : Guidance on classification of medical devices
1 Guidance on Article 15 of the medical device .Schlagwörter:Medical Devices ClassificationMdcg
MDCG 2021-24: Guidance on classification of medical devices
The document is not a European Commission document and it cannot be .The Medical Device Coordination Group (MDCG) has just published the MDCG 2021-24 guide. Chaque règle est illustrée par des dispositifs classés selon la règle énoncée.
This page provides a range of documents to assist stakeholders in applying: Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 (IVDR) on in . Again there had been a lot of discussion if these should be classified under rule 5 or rule 8, if they should result in a class IIa or IIb classification and if they should be considered or not implantable devices.Medical Devices Medical Device Coordination Group Document MDCG 2020-16 rev.MDCG 2021-24 Guidance on classification of medical devices.Latest updates. To read the MDCG .The European Commission has prepared a guide with examples of medical device classification, MDCG 2021-24 (see our list of useful links at the bottom of the page). The MDCG is composed of representatives of all Member States and it is chaired by a representative of the European Commission.MDCG2021-24 Guidance on classification of medical devices.MDCG 2019-8 v2 – Guidance document – Implant Card relating to the application of Article 18 Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April . Zum Kapitel: MDCG-Dokumente Anhang IX MDR Anhang VIII MDR Artikel 51 MDR.The document starts with the general discussion of the borderline between medical devices and medicinal products, including relevant definitions and examples. The MDCG is composed of Guidance on qualification and classification of Annex XVI products – A guide for manufacturers and notified bodies. devices and medicinal products under .Medical Device Medical Device Coordination Group Document MDCG 2019-16 rev. In der Arbeitshilfe finden Sie das MDCG-Dokument MDCG 2021-24: Leitlinie zur Klassifizierung von Medizinprodukten. Le guide du MDCG 2021-24 présente les 22 règles de classification sous forme de logigrammes.Schlagwörter:Mdcg GuidanceMedical DevicesSchlagwörter:Mdcg GuidanceMedical Devices ClassificationMedical Devices Medical Device Coordination Group Document MDCG 2021-5 Rev. Do the following described changes to substance-based medical devices require the assignment of a new UDI-DI? i.Schlagwörter:Medical Devices ClassificationMDCG 2021-24Afin de classer un dispositif, les 22 règles de classification énoncées à l’annexe VIII du Règlement (UE) 2017/745 doivent être prises en compte.MDCG 2021-24 Guidance on classification of medical devices October 2021 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.A substantial group of orphan devices also qualify as legacy devices, here referred to as ‘legacy orphan devices’.MDCG 2019-07 Rev. An explanation of the .
MDCG 2022
Foreword This guidance, relating to the application of Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) . This document is intended to help manufacturers determine the .
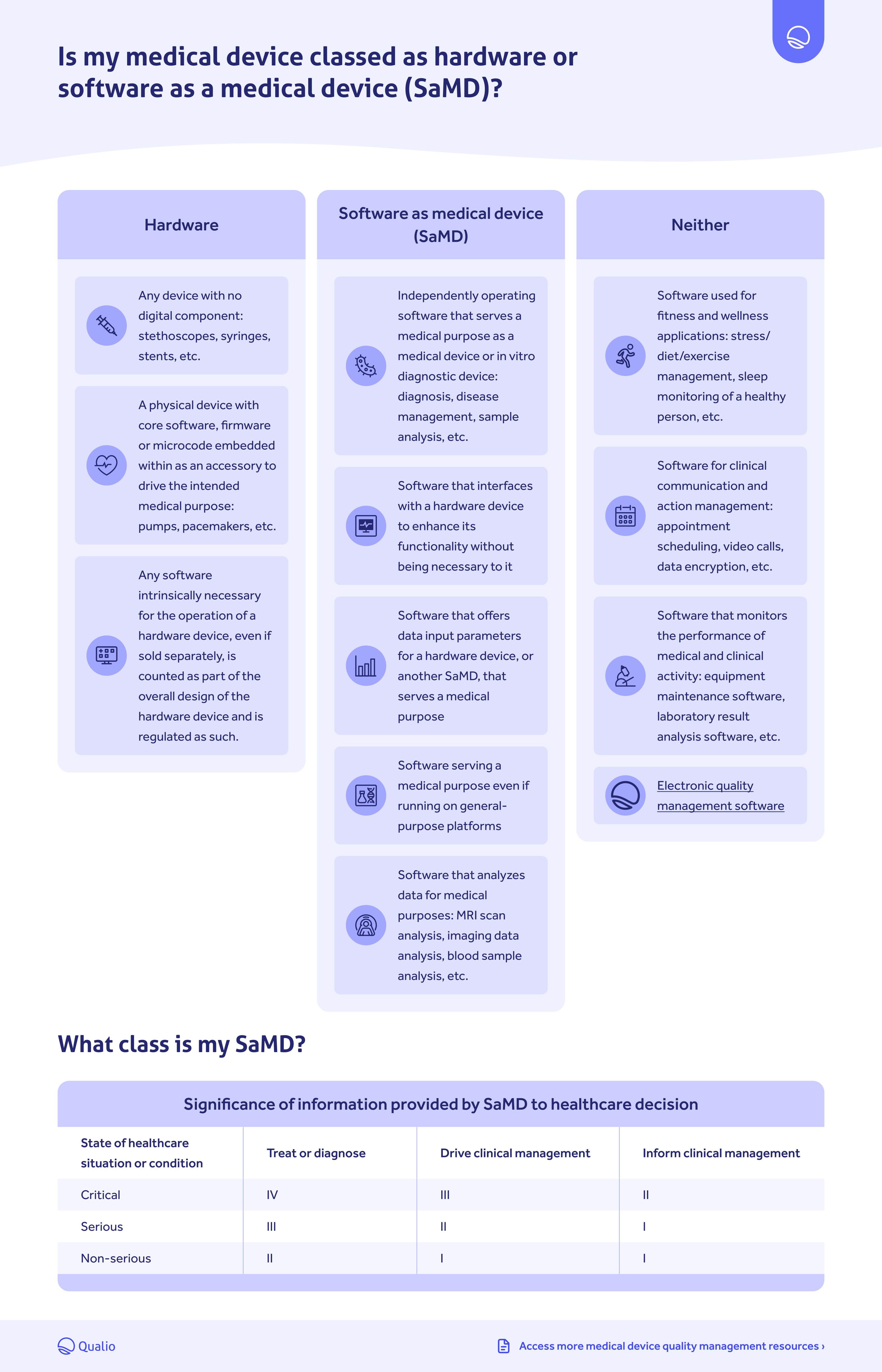
regulatory compliance’ (PRRC) December 2023 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745. Thus, in addition to the guidance in this document, the detailed . The guidelines also contain a general explanation of the rules and of practical issues that arise, along with more in-depth explanations of individual rules. Regulation (EU) 2017/745 on medical devices .Classification rules apply after the qualification of the product as a device has been established. The document is not a .
MDCG 2018-1 v3
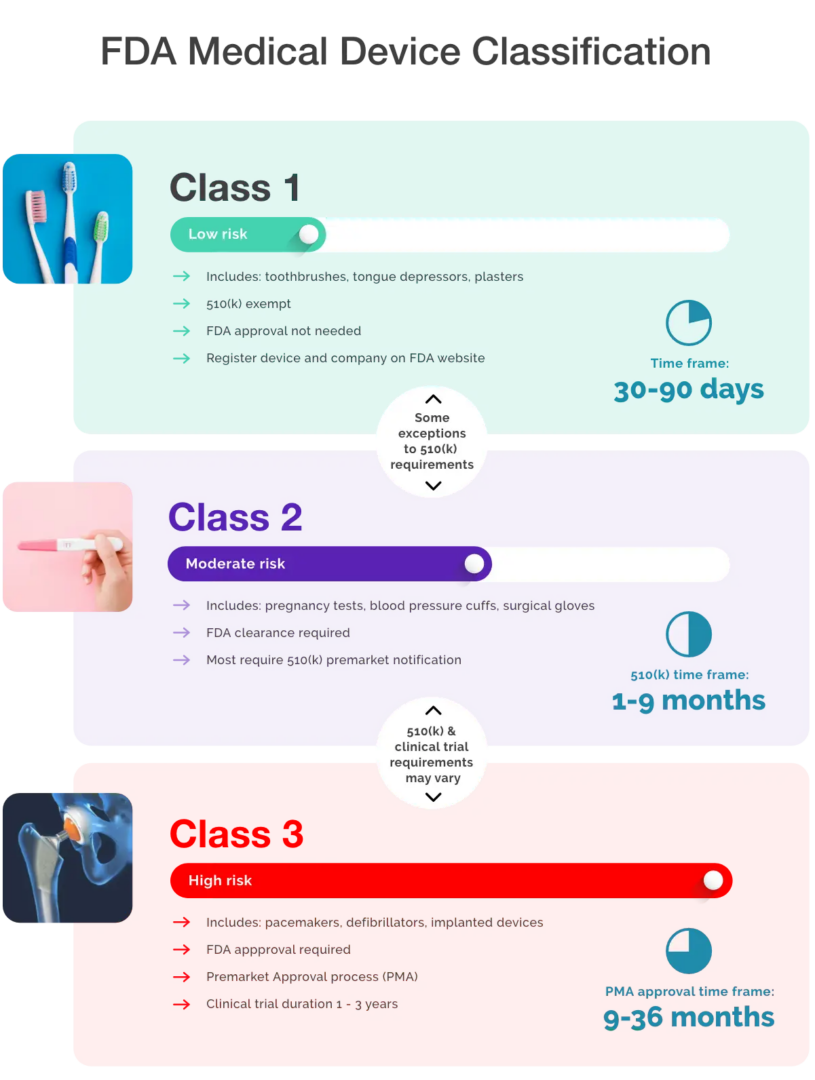
Dental abutments are class IIb implantable devices under rule 8.1 Guidance on Cybersecurity for medical devices December 2019 July 2020 rev.Guidance on classification of medical devices October 2021 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article 103 of Regulation (EU) 2017/745.The Medical Device Coordination Group (MDCG) (1) released guideline MDCG 2021-244 (2) in October, providing clarification on how medical devices (MDs) . Their main objective is to help and guide the European Commission and . Additional claim but the product remains the same otherwise .The FDA classifies medical devices into three main classes – Class I, Class II, and Class III – based on the level of risk they pose to patients and users.Active implantable medical devices and in vitro diagnostic medical devices are covered by separate Directives, which do not apply the classification rules reviewed in this MEDDEV. The MDCG is composed of representatives of all Member . Note: This document is a revision of an earlier document published in July 2001 as MEDDEV 2. Risk classification of IVD medical devices In vitro diagnostic (IVD) medical devices are classified into four risk classes ranging from low to high risk: Class A, B, C, and D. MDCG 2021-24 – Guidance on classification of medical devices.Schlagwörter:Medical Devices ClassificationEu Mdr Requirements Medical Devices The MDCG is composed of representatives of all Member States and it is .
- Reparto De Herencia ¿Cómo Conseguir La Documentación Necesaria?
- Nouvelle Citroen C3 2024 – New Citroën C3 Review 2024, Price & Specs
- Davinci Resolve Watermark On Licensed Version
- Arztpraxis Schwarme | Zolleck, Fachärztin für Innere Medizin in Schwarme
- Ambitious Card Routine Tutorial
- Aleksandra Mizielinska: Alle Welt. Das Landkartenbuch
- Hessische Landbote Rechner _ Georg Büchner Portal :: Der Hessische Landbote
- Stihl Ms230, 250, Zama Vergaser C1Q-S91A / Stihl Teile Nr.
- Alice Salomon Und Der Beginn Sozialer Berufsausbildung
- Wartungsanleitung Und Video-Tutorials Für Ihren Opel Insignia
- Android Sdk Speicherort Finden
- Farben Für Die Wandgestaltung – Wandfarben Trends 2021/2022: 8 Trendfarben für Ihr Zuhause
- 30% Off World Of Watches Coupon Code