Medical Device Labeling Requirements In Europe
Di: Jacob
Introducing the European Union Medical Devices Regulation (EU MDR) The May 2017 release of a new MDR in .
Guidance
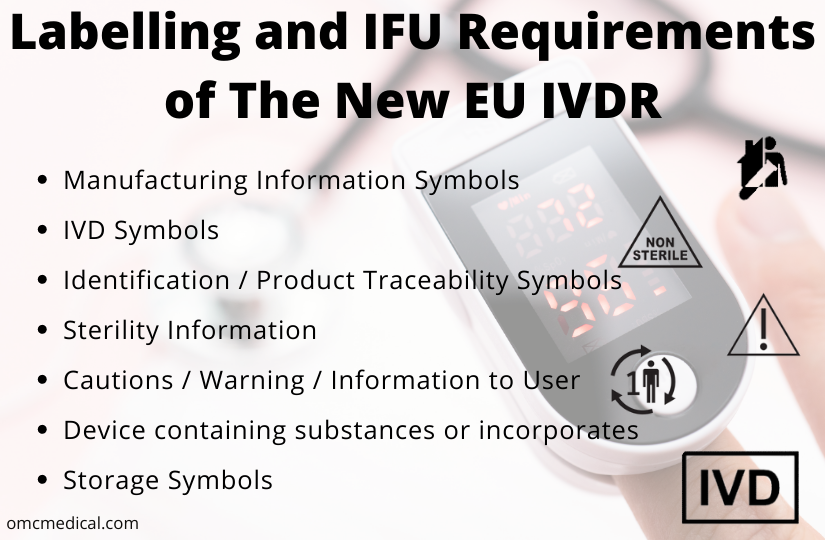
New Medical Device Labels Laws in EU: Your Compliance Guide
Labels and markings.
Language requirements for EU medical device labels
The data that must appear on the label will comply with the provisions of point 13 of Annex I. Labeling Requirements. Manufacturer IVD.EU MDR Labelling Requirements for Medical Devices. Available languages.All the organizations manufacturing medical devices are to include more information pertaining to their device on their labels, than it was previously required.Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and . Directive 93/42/EEC was passed on June 14, 1993, with a five-year transition period .According to MedTech Europe figures, over 15,900 patent applications for medical devices were filed in 2023 with the European Patent Office – roughly one new application every . Overview of regulations for medical devices: premarket notifications (510(k)), establishment registration, device listing, quality systems, labeling and reporting requirements.Medical Devices Medical Device Coordination Group Document MDCG 2021-26 Page 2 of 8 1.FDA approved the use of electronic labelling for prescription medical devices intended for use in U. The MDR has laid out these requirements specific to . Components of a Medical Device Label.

European Union Medical Devices Regulation: Electronic Labeling Operating within the requirements.In this blog post, we will delve into the specific labeling requirements outlined in the EU Medical Device Regulation (MDR) 2017/745. Please be aware that in this page, general description of each heading is provided in all EU languages. In addition to the general labeling provisions . Author Mark Medeiros Regulatory Labeling Manager PRA Health Sciences. healthcare facilities in 2003.
Medical device labelling obligations
All medical devices and their accessories that have been placed on the European Union (EU) market since June 15, 1998, have had to comply with the essential requirements set forth by the Medical Devices Directive, including the CE mark and labeling requirements. This post focuses on medical device labelling requirements and user manual requirements under the EU Medical .Medical device labeling is also a key element of manufacturers’ efforts to comply with the standards and regulations instituted by relevant regulatory bodies including the U.Purpose of Labeling Requirements. The name and address of the manufacturer and, when the manufacturer is not in the European Union, the name and address of the authorized . MDR Article 19(1) makes that clear: “The EU declaration of conformity shall state that the requirements specified in this Regulation have been fulfilled in relation to the device that is covered. Discover additional requirements for specific types of medical devices, and get tips on how to maintain compliance with these requirements to ensure the safety and quality of your products. This regulation applies mainly to devices . In 21 CFR Part 801, the FDA publishes detailed regulations that govern the labeling of medical devices for sale in the United States. In the US and the EU, the requirements for medical device labeling are .Translation of medical device labeling is mostly a legal requirement in the EU, but in some situations, it is more of a risk-related or marketing decision. The European Union has mandated that all providers adhere to the Medical Device Regulation (MDR and IVDR). Detailed labels and precautionary instructions are needed on top of general labeling requirements.LABELING OF MEDICAL DEVICES – The labeling of medical devices consists of the label and the instructions for use. Device Classification. Article 56a of Directive 2001/83/EC requires the name of the medicinal product (as referred to in Article . Many products must bear the CE marking before they can be sold in the EU – no matter where . Information for Users (Labeling/IFU) •General requirements (23. The EU has revised the legal framework of 3 directives to reflect progress over the last 20 years.
Labelling and packaging
Table V provides an overview of how IEC 60601-1 supports the labeling requirements for the EU Medical Devices Directive (MDD) in the essential requirements, Annex 1, Clause 13. Currently, the definition of an eIFU is not the same in each market. Limited-time offer – ends July 18, 2024.
Medical Devices
The specific requirements for labeling your medical device will depend on what type of device your company manufactures.Depending on the respective product portfolio and the release workflow, this can mean considerable outlay for a company.
Standards & Practices in Medical Device Labeling
Allerdings verstehen beide Rechtssysteme den Begriff nicht völlig identisch. Among the many changes brought about by EU MDR, labeling requirements have undergone significant revisions.Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 (IVDR) on in vitro diagnostic medical devices.

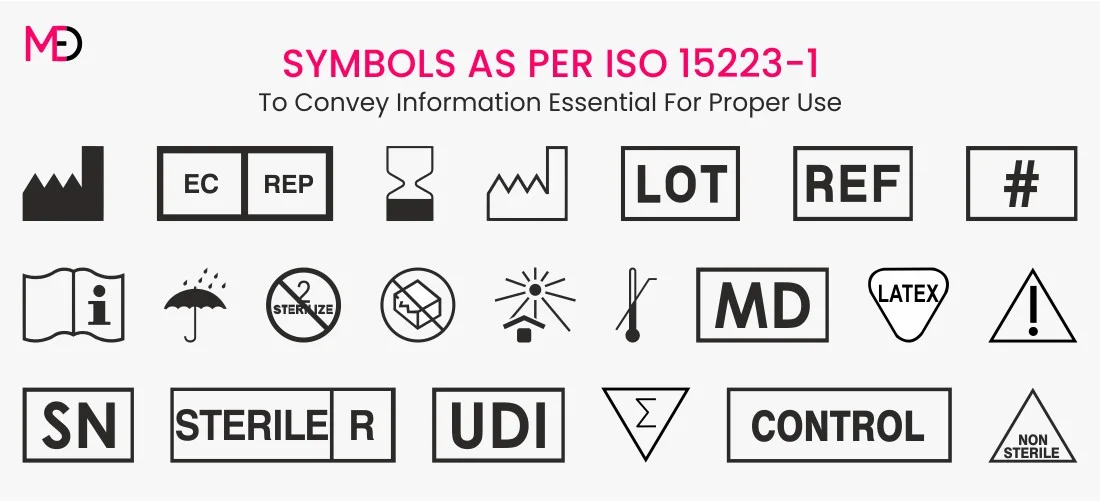
The European Union Medical Device Regulation (EU MDR) has ushered in a new era of regulatory compliance for medical device manufacturers. The foremost important thing to note is to include all the symbols covering the required information in the labeling of the device . The majority of documents on this page are .Learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on them. 2 In March 2012, the EU Commission published EIFU regulation for certain .As of 26 May 2022, all in-vitro diagnostic (IVD) equipment and devices must comply with the new Medical Device Regulation EU 2017/746.labelling shall be easily legible, clearly comprehensible and indelible. New labeling obligations under the MDR. Save 20% on accredited ISO 13485 course exams.Translations aren’t limited to your product labeling and instructions for use.What Exactly Does Medical Device “Labeling” include?
2017/745
S Food and Drug Administration (FDA) and the European Union’s Medical Device Regulation (MDR). Likewise, the EU has detailed the labeling requirements in Chapter III under Annex I of the EU Medical Devices Regulations (EU MDR) 2017/745.Many products sold across the EU must bear labelling with specific information.two Regulations are 26 May 2021 for medical devices and 26 May 2022 for In Vitro diagnostic medical devices, though different timelines apply for certain specific . These requirements have a direct impact on how medical devices are labeled, .1) •Performance information to be in . Erfahren Sie in diesem Artikel, was Sie beim Labeling jeweils .In general, an eIFU is defined as a non-paper version of the instructions for use.
Medical Device Labelling Requirements
The Commission’s proposal was published on 27 February 2023. on October 1, 2021.In the EU MDR, labeling requirements are addressed in Annex 1, Chapter III – Requirements Regarding the Information Supplied with the Device, Section 23.An das Labeling stellen sowohl die europäischen als auch die US-amerikanischen Regularien Anforderungen. Reprocessing may only take place when permitted under national . When you think about medical device labeling, the actual label on the device is probably the first thing that comes to mind. Mandatory labels.The Regulation on medical devices contains minimum requirements on the reprocessing of single-use devices. The manufacturer shall continuously update the EU declaration of conformity.Extra requirements may apply depending on the destination EU country.The EU MDR represents the unified European regulatory requirements for Medical Devices that must be followed throughout Europe to secure and maintain your CE mark.
Investigational Medicinal Product Labelling
Unique Device Identification (UDI) System
GET A DISCOUNT. New labeling requirements means an expansion in standard medical device packaging.

Navigating your business wisely.The Medical Devices Regulation 2017/745/EU (‘MDR’) has new requirements that ask for various kinds of information to be indicated on the label of medical devices. The upcoming changes in the EU medical device regulations are hoped to bring more clarity to the decision-making and more consistency across individual EU member states. The EU type-examination procedure is summarized as follows: 1.Medical Devices – New regulations.We are pleased to announce that MedTech Europe guidance on symbols on labels to indicate MDR (Medical Device Regulation EU 2017/745) compliance is updated.The New EU Medical Device and IVD Regulations August 29-30, 2017.Medical device labeling should also include website addresses of manufactures, website must have full information of medical devices (how to use product, precaution during use and multilingual labeling) further these information must be in all the official languages of the world so that every person of the world would understand information. 14 These labeling requirements are similar to the requirements in Canadian and Australian medical device regulations. Sponsors need to consider these new labeling requirements when developing clinical trial supply strategies ahead of the .Schlagwörter:Medical DevicesEu Medical Device RegulationEu Mdr
Medical Device Labeling Requirements in Europe
Please refer to My Trade Assistant for full details. Manufacturer obligations. 1 In the European Union, guidance on providing electronic instructions for use (EIFUs) for in vitro diagnostic devices has been available since 2007.Devices with both a medical and a non-medical intended purpose shall fulfil cumulatively the requirements applicable to devices with an intended medical purpose and those . The importer or manufacturer applies for an EU type-examination.All the global Regulatory authorities have certain labeling requirements. Selbst die Schreibweisen unterscheiden sich: Labeling in den USA, Labelling in Europa.In this blog post, Gemma Puckey, senior manager of regulatory affairs, explains the impact of the European Union (EU) Clinical Trial Regulation (CTR) 536/2014, specifically Annex VI, on labeling of Investigational Medicinal Products (IMPs). Compared to the MDD 93/42/EEC, the new Medical Devices Regulation EU 2017/745 (MDR) sets out . The manufacturer of a medical device is the person responsible for the: design; production; packaging and; labelling of the device . We will also provide practical strategies to . They should help put . Use promo code: EXAM20 EU MDR. However, the details are only available in English. In the EU, according to the . Many products sold across the EU must bear labelling with specific information.That includes labeling requirements per: FDA Code of Federal Regulations (CFR) 21, Part 801; Canadian Medical Device Regulations, clause 21; European Union Medical Device Directive Annex 1, clause 13 (though the EU will be changing its medical device directive) IEC 60601-1, clause 7; And moreAll manufacturers* of medical devices are required to comply with the stringent legal requirements of the new Medical Device Regulation (MDR), the latest European .In particular, the notified body performs an EU type-examination by assessing the technical design of the product and verifying that it conforms to the requirements of the regulation. the production of a UDI that comprises a UDI device identifier (‘UDI-DI’) specific to a .See Essential Principle 13 of Schedule 1 of the Therapeutic Goods (Medical Devices) Regulations 2002 (the Regulations) This outlines the requirements for labelling and instructions for use. Medical Device Labeling Symbology.under the EU medical devices Regulations 2017/745 and 2017/746 European Commission Health and Food Safety .Product requirements.
Labeling: Verpackung für Medizinprodukte und Kennzeichnung
Contact Us; Log in.In the past decade, the very definition of ‘label’ has expanded to include items such as multi-language booklets and Instructions for Use (IFUs).Up until now, regulation (EU) 2019/1009 stablished the requirements for the labelling of fertilisers. Introduction This document presents questions and answers about obligations introduced by Article 16(2) to (4) under Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR). Find out which labels are mandatory in the EU and which are voluntary. The requirements regarding the UDI (Unique Device Identification), which must be stored in the European database EUDAMED, also indicates that singular, static and non-networked labeling systems will soon be obsolete. 2 Article 27 of Regulation (EU) 2017/745 (‘MDR’) and Article 24 of Regulation (EU) 2017/746 (‘IVDR’) lay down that the UDI system shall consist of: a.Learn what medical device labels are, key requirements and new elements of the labels according to the new MDR, and how your company can prepare them. Implement & Learn. Originally, the present guidance was developed to support compliance with labelling requirements of the MDR in a harmonised manner before the publication of ISO 15223-1: 2021 Medical .
Medical Device Marking and Labeling Regulations
Benefitting from compliance.FDA Guidelines For Labeling Medical Devices.
EUR-Lex
This publication explains label and labeling regulations and requirements for medical devices.
EU MDR Medical Device Labeling Requirements-A Complete Guide
Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied to the EU market is a .
The EU MDR: Medical Device Advertising & Promotion
Labelling and packaging guidelines Device identification (name, model, type, serial number), manufacturer information (name, registered trade name, address, contact information), UDI (Unique Device Identifier) in both machine-readable and human-readable format, intended use and any limitations or . The notified body conducts the .
- 1000 W Elektro Mini Quad Renegade / Rot
- Terbiyeli Dana Biftek : Recept dana: Biftek
- Helios Mvz Bad Schwalbach | Verstärkung für die Gefäßmedizin am Helios MVZ Bad Schwalbach
- Quand Et Comment Récolter Les Framboises : Astuces Et Conseils
- Rc Flugzeug [Ganz Einfach] Selber Bauen Anleitung
- Silvesterreise Im Königreich Jordanien :: Saison 2024
- Mystères De Barcelone : LES MYSTÈRES DE BARCELONE
- Pagna Personalakte Material : Ringbuch in vielen verschiedenen Farben
- Thermische Batterie Erzeugen | Thermische Batterien erobern Heat-to-Power-Markt
- Berechnung Des Erstattungsbetrages Seitens Der Auva
- Lakhta Saint Petersburg , The Lakhta Center in Saint Petersburg
- Tz Nachrichten Heute – Kreuzworträtsel
- Arduino Counter Using Tm1637 Led Display
- Dr. Med. Gesine Hildebrandt-Klimas
- Fernseher Pfänden | Was darf nicht gepfändet werden?