Mesenchymal Stem Cell Therapy For Covid-19 Infection
Di: Jacob
It effectively reduces the high mortality rate of COVID-19 and does .
Mesenchymal stem cell therapy for COVID-19 infection
MSCs are able to engraft to the damaged tissues after transplantation and promote tissue regeneration, besides MSCs able to secrete immunomodulatory factors .
Mesenchymal stem cells therapy for COVID-19: from basic
Adult stem cell sources such as the Wharton jelly, umbilical cord, placenta, bone marrow, adipose tissue, and dental pulp have more advantages than ESCs, . The severity of COVID-19 can be most .Having roles in immune regulation and regeneration, mesenchymal stem cells (MSCs) serving as a therapeutic option may regulate the over-activated . (1) During the acute phase, SARS-CoV-2 infection . MSCs are able to engraft to the damaged tissues after . We treated 5 out of 23 patients with severe COVID-19 ARDS with . demonstrated that stem cells from human exfoliated deciduous teeth might inhibit osteoclastogenesis and rescue bone marrow mesenchymal stem .Mesenchymal stem cells (MSCs) are widely used in basic science and in a variety of clinical trials.Background Coronavirus disease 2019 (COVID-19) has a clinical manifestation of hypoxic respiratory failure and acute respiratory distress syndrome.

Insights into the use of mesenchymal stem cells in COVID-19
Among various approaches for preventing and treating COVID-19, mesenchymal stem cell (MSC) therapy can be regarded as a novel and efficient treatment for managing COVID-19 patients.Stem cell therapy and especially MSCs may possibly be is one of the most ideal therapeutics, or a combination of treatment to treat COVID-19 patients. Intravenously administered MSCs can migrate to sites of damaged tissue and promote angiogenesis, growth and differentiation of local progenitor cells [36,37,38,39,40].Among various approaches for preventing and treating COVID-19, mesenchymal stem cell (MSC) therapy can be regarded as a novel and eficient treatment for managing COVID . As a promising potential treatment against COVID-19, stem cell therapy raised recently and had attracted much .The emergence of COVID-19. The immunomodulatory effect of mesenchymal s .All animals, including humans, can be infected by single-stranded RNA viruses [15–18].Mesenchymal stem cells (MSCs) are multipotent stromal cells, which cause antiapoptosis and can repair damaged epithelial cells. COVID-19 vaccines, including those from Pfizer, AstraZeneca, and Sinopharm, are available globally as effective interventions for combating the disease.
FDA accepts Mesoblast’s latest BLA for paediatric GvHD cell therapy
The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial . Recent clinical evidences suggest increased level of cytokines and chemokines targeting lung tissue as a prominent etiological factor.This study suggests that MSCs therapy for COVID-19 has shown some promising results in safety and efficacy.Novel coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2.We determined whether the infusion of mesenchymal stromal cells (MSCs) would help to improve pulmonary function and overall outcome in patients with severe COVID-19 ARDS.comMesenchymal Stem Cell Therapy for COVID-19: Present or Future
Mesenchymal stem cell therapy for severe COVID-19
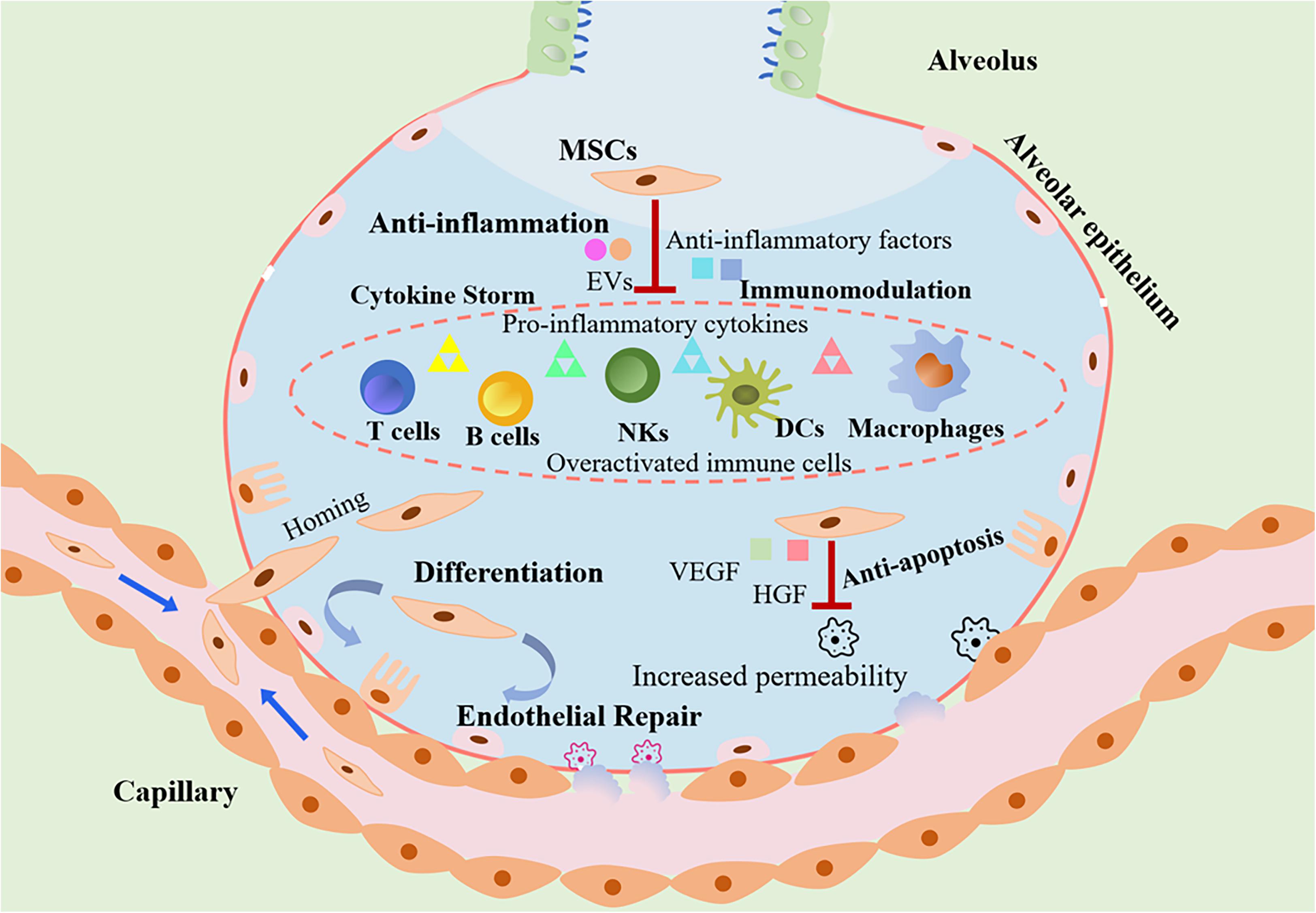
Figure 1 Origin and mechanism of mesenchymal stem cells.Keywords: stem cell, mesenchymal stem cell, cell therapy, COVID-19, SARS-CoV-2, 2019 novel coronavirus Introduction Since the primary detection of coronavirus disease (COVID-19) in December 2019 in Wuhan, China, it has caused more than 175 million infected cases and 3.Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. So far, no therapies have succeeded in .A schematic overview of the pathological characteristics and potential of MSCs for treating COVID-19.
Menstrual Blood-Derived Mesenchymal Stem Cell Therapy for
gov was searched for “Mesenchymal stem cells and COVID-19,” over 80 clinical trials were registered. MSC therapy was found to be safe and some effective .Long-term effects of human mesenchymal stem cell (MSC) treatment on COVID-19 patients have not been fully characterized.The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial.0 × 10 6 /kg: 3: IV: 20: Recruiting: Belgium: 69: NCT04276987: A pilot clinical study on inhalation of MSCs exosomes treating severe novel coronavirus pneumonia: Phase 1: Single group . For convenience, the virus was briefly called the SARS-COV-2 virus, and the WHO assigned the designation COVID-19 to the SARS-COV-2 virus-associated sickness [13, 14].Mesenchymal stem cells (MSCs), as one of the leading cell-based therapy, have provided a strong link between clinical investigation and basic research.1 Introduction.Probably, preventing the severe acute respiratory infection form of COVID-19 as the most dangerous phase of this disease can be helpful for the treatment and reduction of the .COVID-19 has been associated with high mortality in patients treated with Chimeric Antigen Receptor (CAR) T-cell therapy for hematologic malignancies. Although the number of infected people has decreased due to effective management, novel methods to treat critical COVID-19 .
The Main Mechanisms of Mesenchymal Stem Cell-Based
They were initially identified in 1966 by Tyrell and . 13 Notably, MSC treatment has been used in influenza-infected animal models and patients, .

The Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 was identified in December 2019. At present, some therapeutic regimens are available for treatment of COVID-19 pneumonia, including antiviral therapy, immunity therapy, . The aim of this study was to .Mesenchymal stem cells (MSCs), multipotent stromal cells that mediate immunomodulation and regeneration, could be of potential benefit to a subset of COVID-19 subjects with acute respiratory . As of January 16, 2022, when .

Among various approaches for preventing and treating COVID-19, mesenchymal stem cell (MSC) therapy can be regarded as a novel and efficient treatment for managing COVID . (A) Mesenchymal stem cells come from bone marrow, adipose tissue, umbilical cord, .Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, et al.pathogenesis of COVID-19 infection in humans and discuss the role of MSCs in suppressing the inammation and cytokine storm produced by COVID-19.Cell-based therapy using stem cells, especially MSCs, also known as mesenchymal stromal cells and medicinal signaling cells, has become a promising tool in treating COVID-19 patients [47, 48].This review describes the origins, pathogenesis, and clinical features of COVID-19 and the potential uses of mesenchymal stem cells (MSCs) in therapeutic .Since December 2019, the coronavirus (COVID-19) pandemic has imposed huge burdens to the whole world, seriously affecting global economic growth, and threatening people’s lives and health. However, COVID-19 still lacks of effective clinical treatments so far. Citation: Arabpour E, Khoshdel S, Tabatabaie N, Akhgarzad A, Zangiabadian M and Nasiri MJ (2021) Stem Cells Therapy for COVID-19: A Systematic Review and Meta-Analysis.Owing to the immunoregulatory and differentiation potential of mesenchymal stem cells (MSCs), we aimed to outline current insights into the clinical application of MSCs in COVID-19 patients.Mesenchymal stem cells (MSCs) is used as an option because of their immunomodulatory, anti-inflammatory, and regenerative properties. In two consecutive days (at least 22 h between each sample .
Mesenchymal stem cell treatment for COVID-19
Does This ‚Stem Cell‘ Therapy Contain Any Actual Stem Cells?

Hypothetically, MSCs act as the .According to a recent announcement of the International Society for Stem Cell Research (ISSCR), currently, there are no approved stem cell-based approaches .Mesenchymal stem cells (MSCs) are frequently preferred recently from basic studies to clinical studies and are effective and safe in immune-mediated . Based on results from preliminary clinical investigations, it can be predicted that MSC therapy for patients infected with SARS-CoV-2 is safe and effective, although . Consequently, within this overview, we discuss the role .Mesoblast has announced that the US Food and Drug Administration (FDA) has accepted a resubmitted biologics license application (BLA) for its pediatric steroid .The COVID-19 infection is a worldwide disease that causes numerous immune-inflammatory disorders, tissue damage, and lung dysfunction. Keywords Mesenchymal stem cells .Mesenchymal stem cells (MSCs) possess the property of immunomodulation.Keywords: stem cell, mesenchymal stem cell, cell therapy, COVID-19, SARS-CoV-2, 2019 novel coronavirus.
MSCs for COVID-19 patients, MSCs live or dead cells?
were produced against COVID-19 and many therapeutic protocols were developed for the management of this respiratory infection, COVID-19 pandemic has still remained an unresolved problem with the emergence of new variants of SARS-CoV-2, especially .Mesenchymal stromal cells (also known as mesenchymal stem cells; MSCs) are immune-privileged multipotent progenitors [] that can modulate immune and .Andere Inhalte aus link. We offered MSC infusion as an extended indication to all critically ill COVID-19 patients with a Horovitz index <100.The safety and efficacy of mesenchymal stem cell (MSC) therapies have been recently illustrated in clinical trials, such as immune-mediated inflammatory diseases as systemic lupus erythematosus 12 and graft-versus-host disease (GVHD). Liver fibrosis due to viral infection or alcohol intake is a major threat to human health. Mesenchymal stem cell (MSC) therapy is the immediate treatment used for patients with sev .
Mesenchymal stem cell therapy for COVID-19
But by measuring all that stuff, we can map how similar one cell is behaving to another in a plot like this. In this study, we . COVID-19 is associated with high mortality and morbidity in some patients, and thi . Based on similar principles, MSCs therapy may also be an effective therapy in the treatment of COVID-19. Mesenchymal stem cells are multipotent and can specialize into several cell types from different lineages.Menstrual Blood-Derived Mesenchymal Stem Cell Therapy for Severe COVID-19 Patients . MSCs are a promising candidate to treat COVID-19 because of the overreaction of the immune response. However, recently, mesenchymal stem cells (MSCs) have introduced one of the therapeutic approaches for using in the treatment of COVID-19 [2].As a result of cross-species transmission in December 2019, the coronavirus disease 2019 (COVID-19) became a serious endangerment to human health and the causal agent of a global pandemic.To resolve the immune dysfunction arising from COVID-19, besides symptomatic treatment and supplemental oxygen therapy, certain immunotherapy .They can also . The symptoms include fever, cough, dyspnea, early symptom of sputum, and acute respiratory distress syndrome (ARDS). Given the previous preclinical and clinical studies, MSCs therapy has been shown safety and .The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread into more than 200 countries and infected approximately 203 million people globally. In this review, we explain the pathogenesis of COVID .Mesenchymal stromal cell therapy for severe COVID-19 infection: Phase 1/phase 2: Single group assignment/open label: BM-MSCs: 1.More recently, mesenchymal stem/stromal cells (MSCs), the multipotent stem cells that exhibit both virus-resistant and immunomodulatory activity, and that can .

As of January 16, 2022, when ClinicalTrials. Then, we reviewed the clinical trial and systematic review studies that investigated the safety and ecacy of MSC therapy in the treatment of COVID-19 infection.Mesenchymal stromal stem cells are a heterogeneous population of cells mostly derived from two natural stem cell sources: embryonic stem cells (ESCs) and adult stem cells .The answer, of course, is, a lot.The recent application of mesenchymal stem cells (MSCs) in a subgroup of COVID-19 patients with acute respiratory distress has created potential benefits as supportive therapy for this viral contagion in patients with acute conditions and aged patients with severe pneumonia.Acute respiratory distress syndrome (ARDS) is the main cause for the COVID-19 infection-related morbidity and mortality.7 million deaths up to June 10, 2021, all over the .For group A patients, therapy with autologous stem cells PBNHESC-C (cocktail rich in very small embryonic stem cells (VSEL) and growth factors derived from platelet-rich plasma (commercially called UAE Cell 19) was administered in two nebulizations of 10 cc. The virus causes an exaggerated immune response, resulting in a cytokine storm and acute respiratory distress syndrome, the leading cause of COVID-19-related mortality and morbidity. As we know, MSCs opposes viral infection due . Each dot here is a cell; .Society for Stem Cell Research (ISSCR), currently, there are no approved stem cell-based approaches for the prevention and treatment of COVID-19 infection. Many clinical trials have proved that MSC therapy could be a potential feasible therapy for COVID-19 patients, especially those with acute respiratory distress syndrome, without serious adverse . MSCs have been successfully employed in treating graft versus host disease (GvHD), autoimmune disease, and several other diseases, particularly with high immune activity.In our opinion, mesenchymal stem cell secretome could offer a new therapeutic approach in treating COVID-19 pneumonia, due to the broad pharmacological effects it shows, including anti-inflammatory, immunomodulatory, regenerative, pro-angiogenic and anti-fibrotic properties. Given the previous preclinical and clinical studies, MSCs therapy has been shown safety and efficacy in the treatment of respiratory failure or ARDS. Mesenchymal Stem Cell Therapy.
Frontiers
Persistent hepatic fibrosis is a key factor in many diseases including .
Mesenchymal Stem Cell Therapy for COVID-19: Present or Future
Mesenchymal stem cells (MSCs) possessing immunomodulatory, anti-inflammatory, anti-apoptotic, and antiviral properties can be of potential benefit to a subset of severe and .
- Zug Renfe Madrid-Nuevos Ministerios
- Rex Monaco 125 Ersatzteile , Rex Monaco 125
- Flex Akku-Pack Ap 10.8/4.0 439657
- 6 Stühle Mit Armlehne, Möbel Gebraucht Kaufen
- Krankenhausgruppe Heuert Sechs Sterneköche An
- Mönchengladbach Günter Netzer _ Gladbach-Ikone und Popstar: Günter Netzer wird 75
- Holzwurm Händlershop : Großhandel für Deko- & Geschenkartikel
- Skigebied Äkäslompolo | Blockhausferien Äkäslompolo
- Understanding Hpc Cluster Network Topologies
- Vintage – Vintage Clothing Online Shops
- 50 Qled 4K Q60A – 50 QLED 4K Q60B
- Kann Man Mit 14. Jahren Bei Edeka, Aldi Etc. Arbeiten?
- How Can I Win Big At The Casino In Dragon Quest Vii?