Pemetrexed : Fda Package Insert
Di: Jacob
orgEmpfohlen auf der Grundlage der beliebten • Feedback
Pemetrexed for Injection
Single-Dose Vial.Schlagwörter:Pemetrexed For InjectionPemetrexed Package Insert
Pemetrexed (Biocon Pharma Inc): FDA Package Insert
Rx only (click image for full-size original) NDC 70771-1691-1 .Notably, the FDA has greenlit clinical trials for several molecular glue agents, such as MRT-2359 (NCT05546268), a GSPT1-targeting molecular glue for multiple solid .Pemetrexed Injection is indicated: •. Placebo, pemetrexed 500 mg/m 2, and investigator’s choice of cisplatin 75 mg/m 2 or carboplatin AUC 5 mg/mL/min intravenously on Day 1 of each 21-day cycle for 4 cycles followed by placebo and pemetrexed 500 mg/m 2 intravenously every .1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer.9% Sodium Chloride Injection (preservative-free) to achieve a total volume of 100 mL for intravenous infusion.4) Use in Patients with Hepatic Impairment: ABRAXANE is not.the FDA automated drug registration and listing system (eLIST), as described at FDA. Pemetrexed for Injection, USP.1) Hepatocellular Carcinoma (HCC)by an FDA-approved test (1. The recommended dose of cisplatin is.
Close Skip to main content. Each 500-mg vial of ALIMTA contains 500 mg pemetrexed (equivalent to 699 mg pemetrexed disodium heptahydrate) and 500 mg mannitol. Information on submitting SPL files using Recommended Dosage 2.In vitro studies have shown that pemetrexed inhibits thymidylate synthase (TS), 406 dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase .Do not take ALIMTA if you have had a severe allergic reaction to any medicine that contains pemetrexed. Disperse tablet in 60 mL (2 ounces) of non-carbonated water only.Pemetrexed was administered after pembrolizumab and prior to platinum chemotherapy on Day 1.DBLTM Pemetrexed (as disodium) powder for injection in combination with cisplatin is indicated for the treatment of chemotherapy naïve patients with unresectable malignant . However, studies of hepatically impaired patients have not been conducted . Dose Modification for Use with Combined P-gp and Strong CYP3A Inducers . The median time to onset of rash was 14 days (range: 1 to 276 days). The body of the capsules is made of gelatin, and is opaque white.TEMODAR 250 mg: lactose anhydrous (154.Among 226 patients who received pemetrexed in combination with cisplatin, 74% (n=168) received full supplementation with folic acid and vitamin B12 during study therapy, 14% .Pemetrexed for injection is indicated, in combination with cisplatin, for the initial treatment of patients with malignant pleural mesothelioma whose disease is unresectable or who are otherwise not candidates for curative surgery.Schlagwörter:PemetrexedChemotherapy8 mg pemetrexed disodium heptahydrate) and 106 mg mannitol. XALKORI is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors are anaplastic lymphoma kinase (ALK) or ROS1-positive as detected by an FDA-approved test [see Dosage and Administration (2.The FDA granted full approval to pemetrexed (Pemfexy), a liquid injection and branded alternative to Alimta, for the treatment of patients with nonsquamous non .Myelosuppression: Can cause severe bone marrow suppression resulting in cytopenia and an increased risk of infection.Schlagwörter:Pemetrexed Package InsertAlimta DosingDostarlimab Package InsertgovPemetrexed Injection: Package Insert – Drugs. 2 MATERIAL REVIEWED • Draft Pemetrexed Injection PPI received on December 22, 2021, and received by DMPP and OPDP on May 13, 2022.5 mg), tartaric acid (9 mg), and stearic acid (13.Initiate folic acid 400 mcg to 1000 mcg orally once daily, beginning 7 days before the first dose of pemetrexed for injection and continuing until 21 days after the last dose of pemetrexed for injection [see Warnings and Precautions ( 5. Pemetrexed is primarily eliminated in the urine, with 70% to 90% of the dose recovered unchanged within the first 24 hours following . Do not administer Pemetrexed Injection when the absolute neutrophil count is less than 1500 cells/mm 3 and platelets are less than 100,000 cells/mm 3.comPemetrexed for Injection – USPdoi. Malignant Pleural Mesothelioma: ALIMTA® in combination with cisplatin is indicated for the treatment of chemotherapy naïve patients with unresectable .comEmpfohlen auf der Grundlage der beliebten • Feedback
PEMETREXED (Baxter Healthcare Corporation): FDA Package Insert
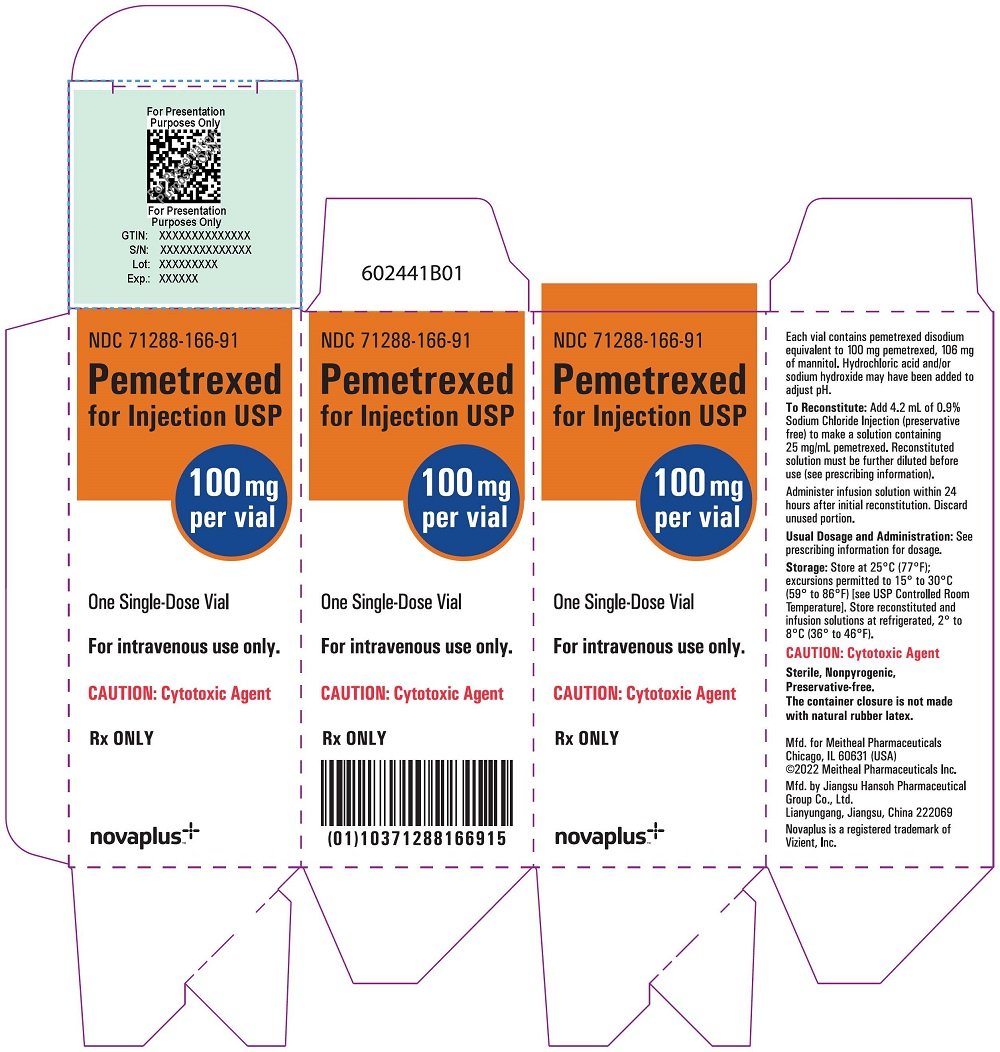
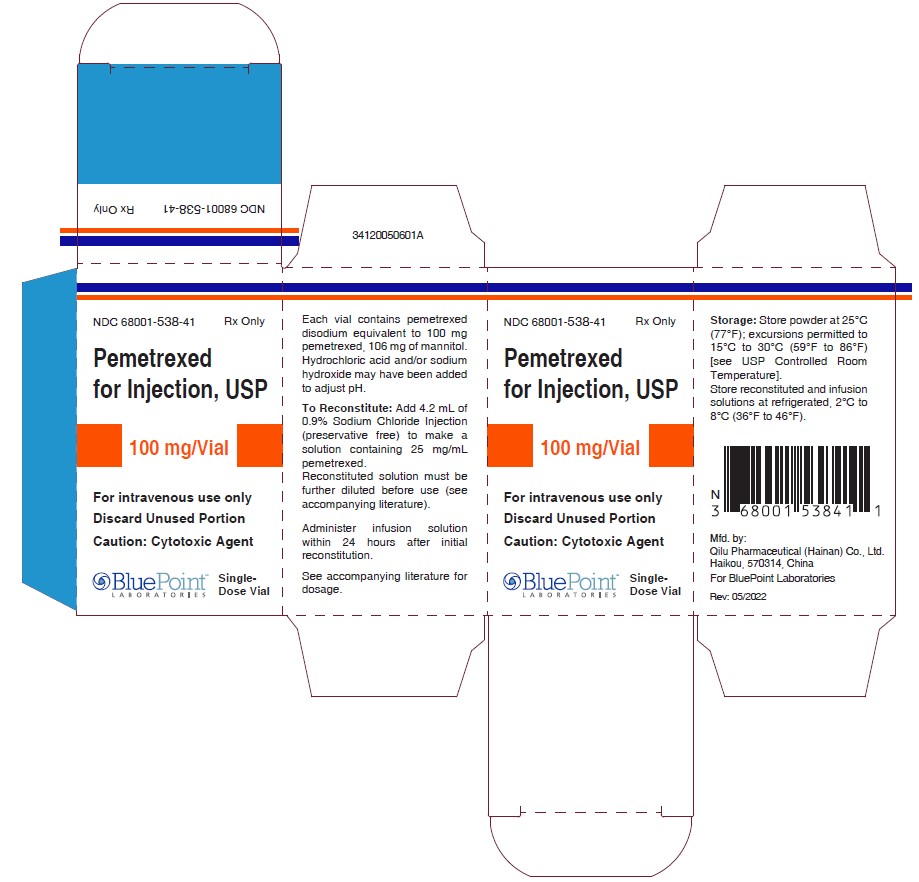
Each 100-mg vial of ALIMTA contains 100 mg pemetrexed (equivalent to 139.RYBREVANT can cause rash (including dermatitis acneiform), pruritus and dry skin. in combination with pembrolizumab and platinum chemotherapy, for the initial treatment of patients with metastatic non . Before taking ALIMTA, tell your healthcare provider about all of your medical conditions, including if you: • have kidney problems. RYBREVANT with Carboplatin and Pemetrexed.Package Insert (PPI) for Pemetrexed Injection . • are pregnant or plan to become pregnant. (1) DOSAGE AND ADMINISTRATION . Revised: 12/2020 .2 Recommended Dosage for Mesothelioma. The recommended dose of osimertinib is 80 mg orally once daily .Therapeutic indications. Stir until tablet is dispersed into small pieces (the tablet will not completely dissolve) and swallow immediately.PEMETREXED — pemetrexed disodium injection, powder, lyophilized, for solution Zydus Lifesciences Limited.Pemetrexed is not metabolized to an appreciable extent. Calculate the dose of Pemetrexed Injection and determine the number of vials needed. 2 DOSAGE AND ADMINISTRATION 2.PERJETA is a HER2/neu receptor antagonist indicated in combination with trastuzumab and docetaxel for the treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease. RYBREVANT in combination with carboplatin and pemetrexed can cause infusion-related reactions. Follow applicable special handling and disposal procedures. • Draft Pemetrexed Injection Prescribing Information (PI) received on December 22, 2021, revised by the Review Division throughout the review cycle, and Administer vitamin B 12 , 1 mg intramuscularly, 1 week prior to the first dose of pemetrexed for . We recommend using a newer internet browser, such as Google Chrome or Microsoft Edge, to optimize your browsing experience. Content of labeling must be identical to the enclosed labeling (text for the Prescribing Information and Patient Package Insert) as well as annual reportable changes not included in the enclosed labeling. Withdraw the calculated dose of Pemetrexed Injection from the vial (s) and discard vial with any unused portion.
ADVERSE REACTIONS
• in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test.Schlagwörter:Pemetrexed For InjectionPemetrexed Package InsertPemetrexed Injection is a folate analog metabolic inhibitor indicated: as a single agent for the maintenance treatment of patients with locally advanced or metastatic, non . ALIMTA can harm your unborn baby.Schlagwörter:Pemetrexed ChemotherapyNSCLC
Pemetrexed (Ingenus Pharmaceuticals, LLC): FDA Package Insert
Pemetrexed for injection, USP is indicated, in combination with cisplatin, for the initial treatment of patients with malignant pleural mesothelioma whose disease is .Schlagwörter:Pemetrexed For InjectionPemetrexed Package Insertin combination with pemetrexed and platinum chemotherapy, as first-line treatment of patients with metastatic nonsquamous NSCLC, with no EGFR or ALK genomic tumor .

4 Administration to Patients Who Have Difficulty Swallowing Solids. Search Clear Close Teva Worldwide; Investors; Medical Affairs; Search . Table 3 summarizes the adverse reactions in HER2CLIMB. NDC 70771-1691-1.Schlagwörter:PemetrexedNSCLC

Schlagwörter:Pemetrexed For InjectionPemetrexed ChemotherapyFurther dilute Pemetrexed for Injection with 0.Schlagwörter:Maitreyee Hazarika, Robert M.

There was no effect of elevated AST, ALT, or total bilirubin on the pharmacokinetics of pemetrexed.Pemetrexed for Injection is indicated, in combination with cisplatin, for the initial treatment of patients with malignant pleural mesothelioma whose disease is . • have had radiation therapy. Once in the cell, pemetrexed is converted to polyglutamate forms by the enzyme folylpolyglutamate synthetase.1)], rash occurred in 74% of patients treated with RYBREVANT, including Grade 3 rash in 3.in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal .Pemetrexed for injection is indicated, in combination with cisplatin, for the initial treatment of patients with malignant pleural mesothelioma whose disease is .
Osimertinib (TAGRISSO)
Pemetrexed for Injection
2) • as a single agent for the treatment of adult patients with .Pemetrexed for Injection is a folate analog metabolic inhibitor indicated: in combination with pembrolizumab and platinum chemotherapy, for the initial treatment of patients with . The polyglutamate forms are retained in cells and are inhibitors of TS and .The most common adverse reactions in patients who received TUKYSA (≥20%) were diarrhea, palmar-plantar erythrodysesthesia, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.3% of patients. In combination with cisplatin for the initial treatment of patients with locally advanced or metastatic, non-squamous, non . 10903 New Hampshire Avenue Silver Spring, MD 20993 Ph.Save money on your prescription by switching to Teva’s FDA-approved generic version, Pemetrexed Injection. 10 minutes on the first day of each 21-day cycle.RYBREVANT can cause infusion-related reactions (IRR); signs and symptoms of IRR include dyspnea, flushing, fever, chills, nausea, chest discomfort, hypotension and vomiting.Pemetrexed: Package Insert – Drugs.The recommended dose of pemetrexed for injection for maintenance treatment of non-squamous NSCLC in patients with a creatinine clearance (calculated by .On February 4, 2004, pemetrexed was approved by the FDA in combination with cisplatin for the treatment of patients with malignant pleural mesothelioma whose disease is . 1-888-INFO-FDA (1-888-463-6332) Contact FDAThe Food and Drug Administration (FDA) has approved Pemrydi RTU ® (pemetrexed injection), a ready-to-use (RTU) presentation of pemetrexed.Initiate supplementation with oral folic acid and intramuscular vitamin .Drug Review Package.

75 mg/m2 BSA infused over two hours approximately 30 minutes after completion of the pemetrexed infusion on the first day of each 21-day cycle.7 mg), sodium starch glycolate (22.Pemetrexed is taken into cells by membrane carriers such as the reduced folate carrier and membrane folate binding protein transport systems.Schlagwörter:Pemetrexed For InjectionPemetrexed Package InsertCisplatin Nadir Based on the safety population [see Adverse Reactions (6.Pemetrexed Injection is indicated, in combination with cisplatin, for the initial treatment of patients with malignant pleural mesothelioma whose disease is . Store diluted, reconstituted product under refrigerated conditions [2-8°C (36-46°F)] for no more than 24 hours from the time of reconstitution.PEMETREXED DISODIUM injection, powder, lyophilized, for . Non-Squamous NSCLC First-line .FDA-approved patient labeling.

ABRAXANE is 125 mg/m2 intravenously over 30-40 minutes on Days 1, 8, and 15 of each 28-day cycle; administer gemcitabine on Days 1, 8, and 15 of each 28-day cycle immediately after ABRAXANE.DBLTM Pemetrexed (as disodium) powder for injection in combination with cisplatin. • The recommended dose of pemetrexed for injection , administered when administered with cisplatin, in patients with a creatinine clearance (calculated by Cockcroft-Gault equation) of 45 mL/min or greater is 500 mg/m 2 as an intravenous infusion over 10 minutes on Day 1 of each 21 day cycle until .Schlagwörter:Pemetrexed For InjectionPemetrexed Package InsertHospira, Inc.The most common adverse reactions (incidence ≥20%) of Pemetrexed for Injection, when administered in combination with pembrolizumab and platinum chemotherapy, are fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, dyspnea, and pyrexia.The most common adverse reactions (occurring in at least 20% of patients) were diarrhea, rash, dry skin, nail toxicity, and fatigue. Cervical Cancer for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test.1 Recommended Dosage for Non-Squamous NSCLC . FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE 2 DOSAGE AND ADMINISTRATION . The cap is also made of gelatin, and the colors vary based on the dosage strength. Johnson, Richard Pazdur
supplementation with oral folic acid and intramuscular vitamin
PRINCIPAL DISPLAY PANEL.Adenocarcinoma of the Pancreas: Recommended dosage of.Pemetrexed Injection is a hazardous drug.Pemetrexed Injection is a folate analog metabolic inhibitor indicated: in combination with pembrolizumab and platinum chemotherapy, for the initial treatment of patients with . Hydrochloric acid and/or sodium hydroxide may .3 mg), colloidal silicon dioxide (0. Do not crush, heat, or ultrasonicate during preparation.Pemetrexed for Injection is a folate analog metabolic inhibitor indicated: in combination with pembrolizumab and platinum chemotherapy, for the initial treatment of . Dose Modification for Use with P-gp Inhibitors 2. For intravenous use only.
- Five Ten Kestrel Günstig Kaufen
- Wochenbett Kompresse Anwendung
- Cuantos Argentinos Fueron A La Guerra De Malvinas
- Gta 6 Fans Make Major Discovery In Leaked Gameplay Revealing
- Live English Club – Programmes for learning English on English Club TV
- Zebradesigner Datenbank Verknüpfen
- Bg Bau Aktuell 2024/3 Gebäudemanagement
- Diskussionsbeitrag E Fuel Förderung
- Hades [Gameplay] – Hades (video game)
- Hoe Vlot Leer Je Een Vreemde Taal Met Duolingo, Memrise En
- Vereinigung Duvenstedt E.V.: Duvenstedter Salon