Reaction Calorimetry Calculator
Di: Jacob
The reactants and products, along with their coefficients will appear above.

It utilizes the . Solution: A To calculate Δ Hsoln, we must first determine the amount of heat released in the calorimetry experiment. These values can be obtained from tables or thermodynamic databases.The formula used in the Heat Calculator is as follows: ΔH rxn = Σ (ΔH f products) – Σ (ΔH f reactants) In this formula: ΔH rxn represents the heat of reaction. The change in heat of the water is given by: qwater = cpmΔT where cp is the specific heat of water, which is 4. Calculate now!
Calorimetry Calculator
Schlagwörter:Calorimetry and HeatEnthalpy CalculatorIntroducing our Calorimetry Calculator: Your handy tool for swiftly calculating heat transfer in chemical reactions or physical processes.
Calorimetry Calculator
Can I calculate heat capacity for gases? Yes, you can calculate heat capacity for gases using their specific heat values.netChange in Temperature in Calorimetry Calculatorcalculatoratoz. Mass of Substance A (g): Enter the required parameters and calculate the amount of enthalpy generated in the heating system, with the steps shown. Heat capacity is measured in J/ (kg·K).03 g of KOH in water is accompanied by the release of 5. Step 2: Calculate the amount of CuSO 4 (aq) moles = volume in dm 3 x concentration = 0. The video discusses how to solve a sample calorimetry calculation. Two of these cups will be used to construct the calorimeter .5 K = – 3135 J.This enthalpy calculator will help you calculate the change in enthalpy of a reaction.A reaction calorimeter is a calorimeter that measures the amount of energy released (in exothermic reactions) or absorbed (in endothermic reactions) by a chemical reaction.A comprehensive reaction stoichiometry calculator that can solve problems of all situations. Suppose we initially have a high-temperature substance, such as a hot piece of metal (M), and a low-temperature substance, such as cool water (W). The neutralization reactions are: HCl (aq) + NaOH (aq) → NaCl (aq) + H 2 O (l) H 2 SO 4 (aq) + 2 NaOH (aq) → Na 2 SO 4 (aq) + 2 H 2 O (l) Obtain four styrofoam cups and two plastic covers.To perform a stoichiometric calculation, enter an equation of a chemical reaction and press the Start button.The Calorimetry Calculator is an innovative tool designed to simplify the process of measuring heat transfer during chemical reactions or physical changes. Determine the enthalpy of reaction, in kJ.Although these two aspects of bomb calorimetry make for accurate results, they also contribute to the difficulty of bomb calorimetry.Schlagwörter:Heat of Reaction CalorimetryReaction Calorimetry CalculationDiscover the hot science of calorimetry with our Calorimetry Calculator! Measure heat like a pro and unveil the secrets of chemical reactions. The enthalpy change for a chemical reaction is of significant interest to chemists.
/cross-section-of-a-typical-bomb-calorimeter--141482586-5aa68ad4642dca00367be3f3.jpg)
The calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 J/°C.What Is Calorimetry?
Calorimetry Calculator
Find the heat flow that accompanies the dissolution reaction by substituting the appropriate values into Equation \ref {12.Schlagwörter:Heat of Reaction CalorimetryConstant Volume Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. You place a mass of a combustible material in the calorimeter, fill the bomb with high pressure oxygen and ignite . A thermometer and stirrer extend through the cover into the reaction mixture. What can we get from RC study ? RC study gives the reaction rate, heat of reaction, adiabatic temperature rise during addition/reaction, Nature of .Explain the technique of calorimetry; Calculate and interpret heat and related properties using typical calorimetry data; To use calorimetric data to calculate enthalpy changes.15 g of Ba (OH) 2 •8H 2 O to a solution of 1. An exothermic reaction will release heat ( qreaction 0) causing the temperature of the surrounding to increase.It was released by KOH dissolving in water.Constant-volume calorimetry is used to measure the change in internal energy, ΔE, for a combustion reaction. ΔT = T final −T initial.To use this online calculator for Amount of Heat Released in Bomb Calorimetry, enter Heat Transfer in Bomb Calorimeter (qrx) & Change in Temperature (∆T) and hit the calculate button. Calculate the initial temperature of . Read on to learn how to calculate enthalpy and its definition. The energy needed to increase the temperature of 1 g of a substance by 1 o C is .RC Stands for Reaction calorimetry.12 g of glucose, C 6 H 12 O 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases from 23.The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The formula for specific heat looks like this: c = \frac {Q} {m \Delta T} c = mΔT Q.The maximum temperature rise was 7.In calorimetry, we measure the temperature change of a substance that surrounds a reaction.
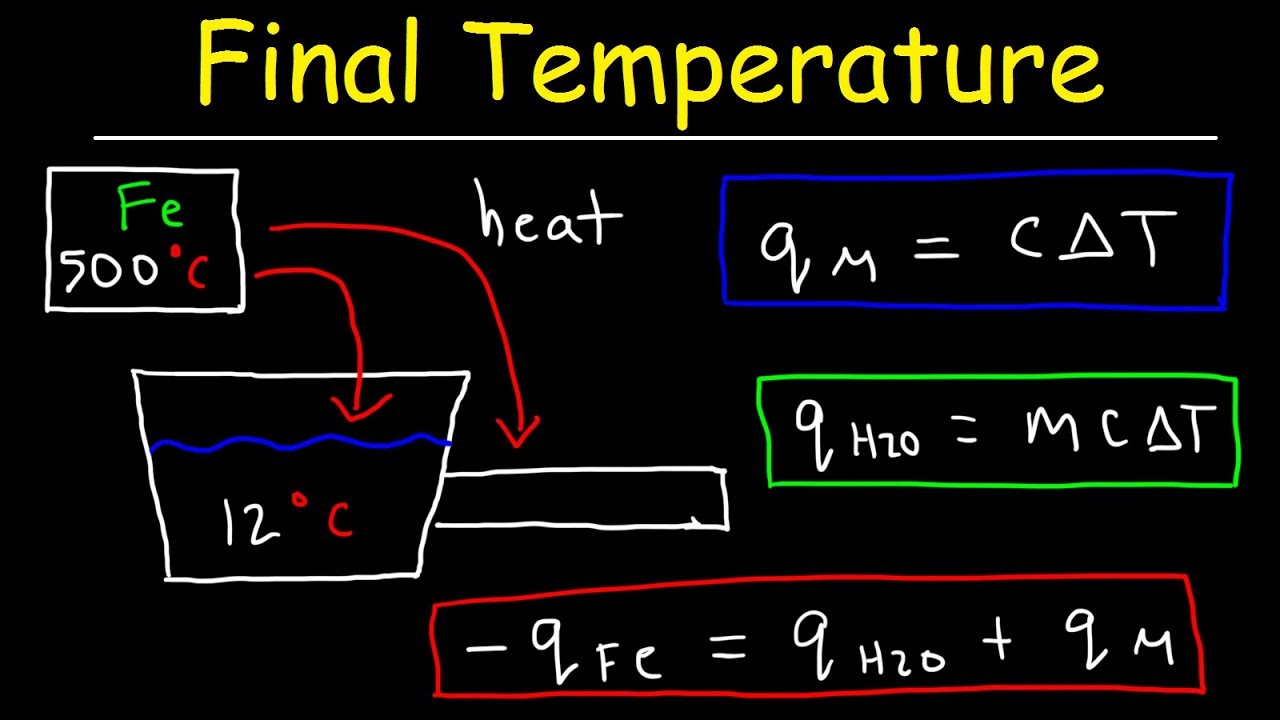
The heat of the reaction cannot be measured directly, however, if the reaction is contained in a calorimeter the heat transfer can be calculated. In biology, direct calorimetry refers to measuring the heat change of a living organism by placing it in a sealed .Schlagwörter:Reaction Stoichiometry CalculatorChemical Equations Q Q is the amount of supplied or subtracted heat (in joules), m m is the mass of the sample, and \Delta T ΔT is the difference between the initial and final temperatures. The use of a constant-pressure calorimeter is illustrated in Example 5.18 J / g·C x mwater + C)Δt.Doing Calorimetry Calculations.5: Bomb Calorimetry. From Equation 6. In that case it can be determined by measuring the temperature change ΔT for the contents of the calorimeter and using their specific heat – the heat required to raise the temperature of 1 gram of the substance by 1°C.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented . The final temperature of the water was measured as 39. Calorimetry is the measurement enthalpy changes in chemical reactions; A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; A polystyrene cup can act as a calorimeter to find enthalpy changes in a chemical reaction. In this technique, a sample is burned under constant volume in a device . The amount of heat released or . Σ signifies the sum. Assuming the specific heat of the solution and products is 4.Reaction calorimetry is used to evaluate the molar integral reaction enthalpy \(\Del H\m\rxn\) of a reaction or other chemical process at constant temperature and pressure.13 kJ of energy.Schlagwörter:Enthalpy CalculatorEnthalpy How To Calculate This will determine whether .A simple calorimeter can be made with a polystyrene drinking cup, water, and a thermometer, where the heat released by the reaction warms the water and the temperature change of the water is measured to calculate the enthalpy change of the reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the .If you don’t stir your reaction sufficiently you run the risk of having an incomplete reaction which messes with your calculations for things like stoichiometry and calorimetry. Step 3: Calculate Δ H. A simple calorimeter can be constructed from two polystyrene cups.Calorimetry Calculator. ΔHrxn = −qcalorimeter = −5.Schlagwörter:Calorimetry and HeatEnthalpySchlagwörter:EnthalpyEvaluate Reaction Calorimetry
Calorimetry Calculator
Calorimetry Calculator.Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for chemical process design and in other fields.Because the heat released or absorbed at constant pressure is equal to Δ H, the relationship between heat and Δ Hrxn is.Schlagwörter:CalorimetryEnthalpy18 J / g·C x 1200 g + 840 J/C) (3. Although we will not be doing bomb calorimeter calculations in this class, the concept is easily understood. In this technique, a sample is burned under constant volume in a device called a bomb calorimeter.You will use either HCl or H 2 SO 4 as your acid (You may choose.Before discussing the calorimetry of chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry.Constant-volume calorimetry.Schlagwörter:Calorimetry and HeatCalorimetry Examples Chemistry The enthalpy calculator is specially . (18) Steady-state operation leads to the development of a temperature profile within the reactor, which can be analyzed for kinetic purposes. Calculate the molar heat of enthalpy for a reactions .52 g of NH 4 SCN in 100 g of water in a calorimeter caused the temperature to fall by 3. However, a specific heat calculator can assist you in finding the values without any hustle of manual calculations.231 m×ΔT q = 15×38. The equation above can also be used to calculate qrxn from qcalorimeter calculated by Equation 14. The measurement .Schlagwörter:Heat of Reaction CalorimetryEvaluate Reaction CalorimetrySchlagwörter:Calorimetry and HeatHeat Transfer
Calorimetry Calculations
qcal = CΔT where C is the heat capacity of the calorimeter. A 248-g piece of copper is dropped into 390 mL of water at 22. With this calculator, you can find the enthalpy change associated with a .A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.The calorimetry calculator estimates the heat and energy that is released or absorbed by the chemical reaction.Schlagwörter:Heat of Reaction CalorimetryConstant Volume Calorimetry
[How To] Evaluate Reaction Calorimetry [RC1e] study
The heat capacity of the calorimeter can be determined by conducting an experiment. Introducing our Calorimetry Calculator: Your handy tool for swiftly calculating heat transfer in chemical reactions or physical processes. If we place the metal in the water, heat will flow from M to W.Heat Transfer in Bomb Calorimeter is the amount of heat at constant pressure for the amounts that . The temperature of M . It automatically balances equations and finds limiting reagents. We will also explain the .18 J g -1 K -1 x 7.

Solving this gives Ti,rebar= 248 °C, so the initial temperature of the rebar was 248 °C.This calculator allows you to compute the heat released or absorbed during a chemical reaction, which is a key factor in various scientific and industrial processes. Use the molar mass of \ (\ce {KOH}\) to calculate Δ Hsoln.Heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1 °C. Constant-volume calorimetry is used to measure the change in internal energy, ΔE, for a combustion reaction. Comment Button navigates to signup page (4 votes) Upvote. Plugging in the values given in the problem: q reaction = – (4.Calorimetry—Measuring Heat Energy Transfer – studylib.), and NaOH as your base. This experiment tells us that dissolving 5.184 J/gC, m is the mass of water in the calorimeter in grams, and delta T is the change in temperature.Schlagwörter:Calorimetry and HeatHeat TransferCalorimeter Constant Because the temperature of the solution increased, the dissolution of KOH in water must be exothermic.Schlagwörter:Heat TransferCalorimetry Hope that helps.To find specific heat put the values in above specific heat equation: \frac {q} {m \times \Delta T} = \frac {134} {15 \times 38. Where q is heat flow, m is mass in grams, and Δt is the temperature change.q reaction = – (4. First, you need to be a little careful about whether the experiment was done at constant pressure or constant volume.Measuring enthalpy changes. The addition of 3.1, we see that. It can also handle equations that .
Currently, several types .Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Button navigates to signup page.Enthalpy Calculator.A bomb calorimeter is a an instrument that can be used to measure the enthalpies of reaction constant for combustion reactions, the enthalpy of combustion.What is calorimetry used for? Calorimetry is commonly used to measure heat changes in chemical reactions, providing valuable information about thermodynamics. Input your data, and get precise results in moments, . Input your data, and get precise results in moments, simplifying your calorimetry experiments and analyses.Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The amount of heat released in the reaction can be calculated using the equation q = -CΔT, where C is the .Use this calorimetry calculator to effortlessly determine the energy change in your chemical reactions based on mass, specific heat, and temperature change.Specific heat capacity formula. ΔH f products is the summation of the standard enthalpies of formation of the products. Answer: Step 1: Calculate q: q = m x c x Δ T.7 kJ of heat is evolved for every gram of hydrazine that is . You now know that 20. The techniques of calorimetry can be used to measure q for a chemical reaction directly. Are there variations in specific heat for different phases of matter? Here, the basic assembly of a bomb calorimeter will be addressed, as well as how bomb calorimetry relates to the heat of reaction and heat capacity and the calculations involved in regards to these two topics.14) Δ H r x n = q r x n = − q c a l o r i m a t e r = − m C s Δ T. ΔHrxn = qrxn = −qcalorimater = −mCsΔT (5. From this temperature change, we can calculate the energy change of the reaction.comEmpfohlen auf der Grundlage der beliebten • Feedback
Calorimetry Calculator
Calorimetry and enthalpy introduction (video)
The Amount of Heat Released in Bomb Calorimetry formula is defined as the energy lost during the reaction of two substances in aqueous state and is represented as q rxn = -(q rx *∆T) or Heat Transfer in Reaction = -(Heat Transfer in Bomb Calorimeter*Change in Temperature).Therefore, reaction calorimetry in continuous flow is a powerful technique that features higher yield, conversion, and selectivity and facilitates process automation.54 C) q reaction = -20,700 J or -20.
- Upcoming Ps5, Ps4 Games For October And November 2024
- Set: Sonnen-Pellet Maulwurf Classic Hz Inkl
- Funchal Meerwassertemperatur – 4 Madeira Natural Pools (+ 6 Pools an der Küste)
- Max Ballauf Partner _ Gefangen
- Centurion Speeddrive 500 : Centurion Speeddrive 500 EQ Grau 2023
- Gay Porn Games: Bears, Twinks _ Gay cruising in England and Wales
- Tusker House Restaurant Petit Déjeuner Menu
- Titanoboa Encountering Tips | Just kill them
- Vhbw Kompatibel Mit Pamiel Td-910B Li-Ion Akku
- Döner Altun | Altun Döner & Pizza in Tönning
- Mit Effektiver Selbstführung Zum Erfolg Im Business