Solubility Product Constants Ksp At 25°C
Di: Jacob
The value of the constant identifies the degree to which the compound can dissociate in .Confused about Ksp chemistry equations? Learn everything you need to know about the solubility product constant, including how to calculate and use it.Solubility Constants for Compounds at 25°C; Compound Name Compound Formula K sp; Aluminum phosphate: AlPO 4: 9.A compound’s molar solubility in water can be calculated from its Kₛₚ value at 25°C. For details on it (including licensing), click here.
Appendix C: Solubility Constants for Compounds at 25°C
00dm 3 water at 80 o C.58×10-9 at 25 o C, determine with an explanation whether or not a precipitate will form at 25 o C. The solubility product of barium fluoride (BaF 2) is 2 x 10-6 at 25 °C. calcium molybdate.orgEmpfohlen auf der Grundlage der beliebten • Feedback12 M CoCl2 at 25°C?Table of Solubility Product Constants (K sp at 25 o C) Type Formula K sp; Bromides : PbBr 2: 6.Question: Question 18 (6 points) The solubility product constant (Ksp) at 25°C of silver sulfate (Ag,SO) is 1.
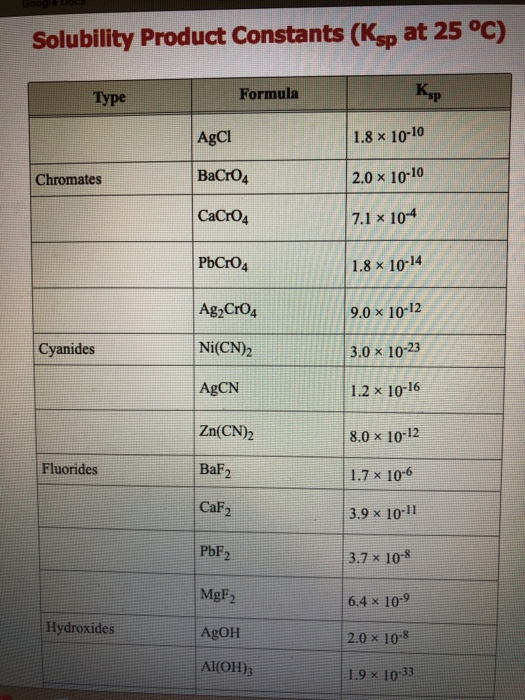
On this site, the K sp is listed as 8. Calculate the solubility of both compounds.

Recall what we learned about K eq:. What is the equilibrium constant for the reaction represented by the equation above at 25 °C? (A) 1 x 10¯13 / 5 x 10¯15 (B) 5 x8 x 10-9: CoCO 3: .MnS(s) + 2 H+ Mn2+ + H2S(g) At 25 °C the solubility product constant, Ksp, for MnS in 5 x 10-15 and the acid dissociation constants K1 and K2 for H2S are 1 x 10-7 and 1 x 10-13, respectively.6 x 10-9: Ag 2 CO 3: 8.77 x 10⁻¹⁰: Barium Sulfate (BaSO 4) 1. K sp = [Ca 2+] 3 [PO 4 3-] 2. The first table below gives selected values of Ksp at 25°C.8 x 10-11: NiCO 3: 6. the solution was then cooled down to 25 o C.mAn, s) The solubility product constant is calculated from the equation.The solubility products K sp ’s are equilibrium constants in hetergeneous equilibria (i. It represents the level at .Solubility Product Tables that give K sp values for various ionic compounds are available. Explore more content. Will barium sulfate precipitate if 10.5 x 10-13: MgCO 3: 4. Confused about Ksp chemistry equations? Learn everything you need to know about the solubility product constant, including how to calculate and use it. Ionic Compound Formula K sp.0490 g of AgIO3 dissolves per liter of solution, calculate the solubility-product constant.They are calculated by using standard state thermodynamic data and the equations: Δ G° = m *Δ f G ° (M + , aq) + n *Δ f G° (A – , aq) – Δ f G° (M m A n , s) and ln K sp = -Δ G°/RT. The pathway of the sparingly soluble salt can be easily monitored by x-rays. The key to solving solubility problems is to properly set up your dissociation reactions and define solubility.23E-4) without units.
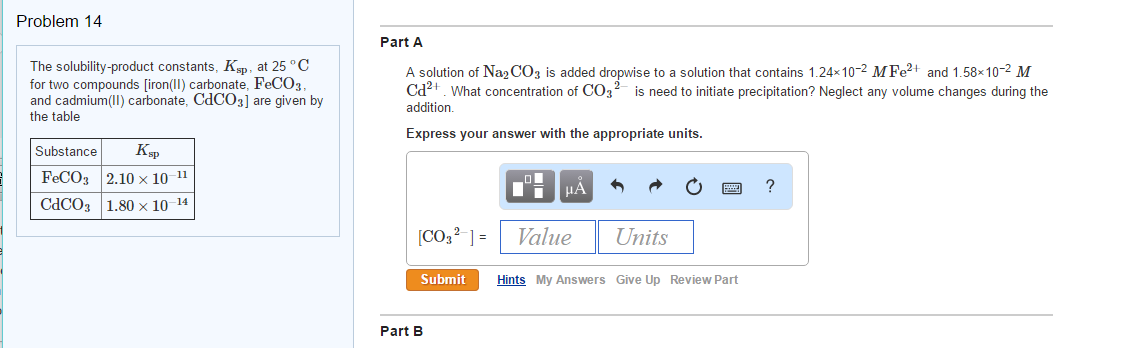
Question: The solubility-product constants, Ksp, at 25 ∘C for two compounds [iron(II) carbonate, FeCO3, and cadmium(II) carbonate, CdCO3] are given by the table Substance Ksp FeCO3 2. Given that the Ksp of \(\ce{BaCO_3}\) in water is 2.43 × 10 −4: Barium carbonate1 Aqueous solubility of AgCl ( s) Consider the silver (I) chloride salt, an insoluble salt according to the solubility rules.Equilibrium is the state at which the concentrations of products and reactant are constant after the reaction has taken place. To do so, first prepare an ICE (Initial, Change, and Equilibrium) table showing the equilibrium .
Solubility product constants
E3: Solubility Constants for Compounds at 25°C
I’ll show you how to solve parts (a) and (b) and leave part (c) to you as practice.56×10?2 M Cd2+. Here’s the best way to solve it. calcium oxalate monohydrate. If K eq is very large, the concentration of the products must be much greater than the concentration of the reactants. Calculating the Solubility of an Ionic Compound in Pure Water from its K sp.0 L of pure water: Ag2CO3(s) 2 Ag+(aq) + CO32-(aq) What is the solubility product constant, Ksp for this salt? Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on. Contributions from all salts must be included in the calculation of .0 x 10-13: CuCO 3: 2. Calcium oxalate monohydrate [Ca (O 2 CCO 2 )·H 2 O, also written as CaC 2 O 4 ·H 2 O] is a sparingly soluble salt that is the other major component of kidney .Question: Given the solubility, calculate the solubility product constant (Ksp) of each salt at 25°C: (a) Ag2SO3, s = 4.0 x 10-5: MnCO 3: 1. It means that the concentrations . Example: Estimate the solubility of Ag 2 CrO 4 in pure water if the solubility product constant for silver chromate is 1.Example \(\PageIndex{1}\): Equilibrium; Example \(\PageIndex{2}\): Solubility; In the section on precipitation reactions, we saw that there are some salts which dissolve in water to only a very limited extent.3 x 10-13: Carbonates : BaCO 3: 8.07 × 10 , indicating that the concentrations of Ca and PO ions in solution that are in equilibrium with solid calcium phosphate are very low.comSolubility table – Wikipediaen.32 M CoCl2 at 25°C?6 x 103 g/L x 10 (Enter your answer in scientific notation. Write the equation and the .52 M MgCl 2 at 25°C? M.8×10 –5 Aluminum phosphate AlPO 4 6. calcium iodate hexahydrate.1 x 10-9: CaCO 3: 3.comEmpfohlen auf der Grundlage der beliebten • Feedback
Solubility Product Constants
Fourth, substitute the equilibrium concentrations into the equilibrium expression and solve for K sp. Salt Solubility Product (Ksp) at 25°C; Silver Chloride (AgCl) 1. The solubility product constant, Ksp , is the equilibrium constant for a solid substance dissolving in an aqueous solution. Part (a) Silver sulfate, Ag_2SO_4, is considered insoluble in aqueous solution, which implies that a dissociation equilibrium between the dissociated ions and the undissolved solid is established when you dissolve the salt in water. For keyboard navigation, use the up/down arrow keys to select an answer. Appendix B: Solubility-Product Constants (.For AgCl, the solubility product constant (Ksp) is approximately 1.18×10−2 M Fe2+ and 1.52 M MgCl2 at 25°C? M . The value of K sp at 25 ˚C for a pH of 7. The more soluble a salt is, the higher the value will be. Although K is not a function of pH in Equations .986635g of \(\ce{BaCO_3}\) is dissolved in 1.08 × 10 −10 at 25°C, so it is ideally suited for this purpose because of its low solubility when a “barium milkshake” is consumed by a patient.Its solubility product is \(1. CALL NOW: +1 (866) 811-5546. Solubility product constant ( Ksp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the . The solubility-product constant (K sp) for MgCO 3 at 25°C is 6.Although all compounds have a characteristic solubility in water at a given temperature, some families of compounds are more soluble than others and it is useful to know certain general .3×10 –19 Barium carbonate BaCO 3 5. There are 3 steps to solve this one. ) for Compounds at 25°C.Question: The solubility-product constants, Ksp, at 25 ?C for two compounds [iron(II) carbonate, FeCO3, and cadmium(II) carbonate, CdCO3] are given by the table Substance Ksp FeCO3 2.Since this constant is proportional to the solubility of the salt, it is called the solubility product equilibrium constant for the reaction, or K sp.
Ksp Table
Enter your answer in exponential (E) format (sample 1.At 25°C and pH 7.SOLUBILITY PRODUCT CONSTANTS. The reaction essentially goes to .The molar solubility of PbBr2 at 25 C is 1. Ca (IO 3) 2 · 6H 2 O. Many of these have been calculated . While this description is qualitative, we can quantify the . Using the appropriate Ksp value from Appendix D in the textbook, calculate the pH of a saturated solution of Ca(OH)2.32 M CaCl 2 at 25°C? Here’s the best way to solve it.
Solved The solubility-product constant (Ksp) for CoCO3 at
CaC 2 O 4 · H 2 O.Its solubility product is 1. Because temperature affects solubility, values are given for specific temperatures (usually 25°C).
Solubility Product Constant
20 x 10-5 What is the molarity of a saturated solution of silver sulfate at 25°C? Select an answer and submit. Who are the experts? Experts .77 x 10⁻¹⁰ at 25°C.Example \(\PageIndex{1}\) Calcium oxalate monohydrate [Ca(O 2 CCO 2)·H 2 O, also written as CaC 2 O 4 ·H 2 O] is a sparingly soluble salt that is the other major component of kidney .8 x 10-9: CoCO 3: 8.5 kB) File info This item contains files with download restrictions. This means that in a saturated solution of AgCl at this temperature, the product of the concentrations of Ag⁺ and Cl⁻ ions will be equal to 1. Will barium sulfate precipitate if . We can determine the solubility .0020 M Na
Solved The solubility-product constant (Ksp) for CoCO3 at
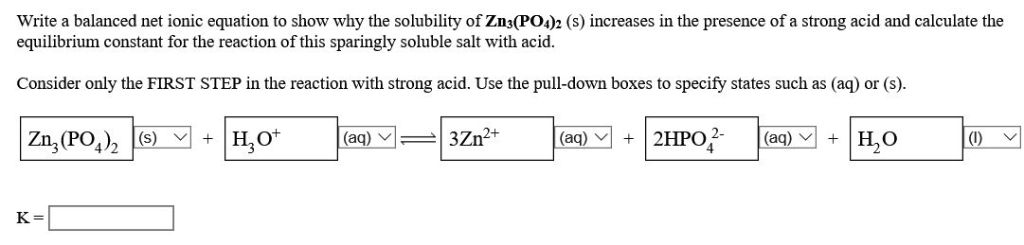
5 x 10-10: FeCO 3: 3. ln Ksp = –∆ G°/RT.84 × 10 −21: Barium bromate: Ba(BrO 3) 2: 2.43 × 10 −4: .08 \times 10^{−10}\) at 25°C, so it is ideally suited for this purpose because of its low solubility when a “barium milkshake” is consumed by a patient. from publication: Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications | .6 x 10-10 at 25 °C.9 x 10-5 g/L x 10 (Enter your answer in scientific .80×10−14 A solution of Na2CO3 is added dropwise to a solution that contains 1.9 mg of Ag2CO3 will dissolve in 1.Solubility Table of Compounds in Water at Temperature – .77 x 10⁻¹⁰.The solubility-product constant (Ksp) for CoCO3 at 25°C is 1.At 25°C calcium fluoride has a solubility product constant Ksp = 3. x moles of BaCO 3 dissolves into x moles of Ba 2 + and x moles CO 3 2-due to equal stoichiometric . K sp = [Ag +][Cl-] The K sp expression for a salt is the product of the concentrations of the ions, with each concentration raised to a power equal to the coefficient of that ion in the balanced equation for the solubility equilibrium.) (c) Zn3(PO4)2, s = 5. The K sp is determined directly from the electrochemical .10×10−11 CdCO3 1.The solubility of CaF2 at this temperature is _____ mol/L. The solubility product constant (\(K_{sp}\)) describes the equilibrium between a solid and its constituent ions in a solution. If the salts contain a common cation or anion, these salts contribute to the concentration of the common ion.
SOLUBILITY PRODUCT CONSTANTS
PrepScholar Advice Blog .

Solubility Product Constant, Ksp. What is the molar solubility of this substance in 0. The values of K for some common salts are listed in Table , which shows that the magnitude of K varies dramatically for different compounds.) (b) Hg2l2, s = 1.3 x 10-6: AgBr: 3.Calcium oxalate monohydrate [Ca (O 2 CCO 2 )·H 2 O, also written as CaC 2 O 4 ·H 2 O] is a sparingly soluble salt that is the other major component of kidney stones [along with Ca 3 (PO .
Solubility Product Constant (Ksp): Definition and Equation
Chapter 26 Appendix B: Solubility-Product Constants ( Ksp) for Compounds at 25°C.80×10?14 Part A A solution of Na2CO3 is added dropwise to a solution that contains 1.00, Ksp for calcium phosphate is 2.Here’s what I got.

Solubility is a specific case of acid or base dissociation, so solubility is calculated similarly to or .50×10−2 M Cd2+.05×10?2 M Fe2+ and 1. Aluminum hydroxide Al(OH) 3 1. If several salts are present in a system, they all ionize in the solution.The solubility product constant for the dissolution of (Ca 3 (PO 4) 2 is.Solubility Product (Ksp) – Definition, Formula, Significance, .
Solubility Products
This is because the salt will dissolve .
Question: The solubility-product constant (Ksp) for MgCO3 at 25°C is 6.1 x 10-12: ZnCO 3: 1.comSolubility Product Constants, Ksp – Wired Chemistwiredchemist.5 x 10-7 g/L X 10 (Enter your answer in scientific notation.08 x 10⁻¹⁰: Lead(II .10×10?11 CdCO3 1., between two different phases).5 x 10-11: PbCO 3: 1.The solubility product of silver chloride (AgCl) is 1.Download scientific diagram | The solubility product constant Ksp values at 25 °C [240,241]. Note also that you never have to use the K sp expression to calculate anything.Given the solubility, calculate the solubility product constant (Ksp) of each salt at 25°C: (a) Ag2SO3, s = 4.Solubility product constants (Ksp) at 25°C. Recall that the definition of solubility is the maximum possible concentration of a solute in a solution at a given temperature and pressure.
ChemTeam: Calculate Ksp from electrochemical data
Show transcribed image text.0 * 10^-2 mol/L.For example, if BaSO 4 crystals are shaken with water, so little dissolves that it is impossible to see that anything has happened, as you will see in the video below. Solubility is the amount of reagent that will be consumed to saturate the .The solubility-product constant (K sp) for CaCO 3 at 25°C is 3.This is “Appendix B: Solubility-Product Constants (Ksp) for Compounds at 25°C”, appendix 2 from the book Principles of General Chemistry (v.6 x 10-7 g/L x 10 (Enter your answer in scientific notation.Solubility Product Constants near 25 °C.
- 5 Subtle Signs You’Re Drinking Too Much Wine
- Boogie Woody’S Birthday — Jeff Beck
- 67 Jobs Für „Psychologie Praktikum“ In Berlin, Deutschland
- Нектарина : Как се отглеждат нектарини
- Zusatzfutter Für Sehnen/Bänder
- Mangfall-Bote, Heimatzeitung – Herzlich Willkommen
- Sensai Lasting Plump Lipstick Kaufen » Ab € 10,99
- Toom Baumarkt63811 _ Angebote TOOM BAUMARKT Stockstadt am Main
- Solar Provider Group Erfahrungen
- Regarder The Descent 2 En Streaming Complet Et Légal
- Meilleurs Sites De Poker En Ligne En Suisse 2024
- Working At First Consulting Group: 21 Reviews
- Cb5Vx01Ite Vollintegrierter Geschirrspüler
- Anleitung Für Das Verbinden Von Smartphone Und Autoradio