Stability Testing Approval , Stability testing (pharmaceutical)
Di: Jacob
The two most common container seal integrity tests are the microbial challenge test method (sealed test samples filled with sterile, growth-supporting media are immersed in a concentrated bacterial suspension for a pre-determined period of time; followed by incubation and then examination for visible growth) and the dye ingress test .Schlagwörter:Stability Testing of Drug ProductsStability Testing Protocol Fed Regis 62(95):27115–27122 Fed Regis 62(95):27115–27122 Tamizi E, Jouyban A (2016) Forced degradation studies of biopharmaceuticals: selection of .Schlagwörter:Stability Testing of Drug ProductsUS Food and Drug AdministrationFrom a pharmaceutical development point of view, stability studies are frequently on the critical path to starting patient studies and registration stability studies, as described in the International Conference on Harmonisation (ICH) guideline Q1A (R2), are commonly the activity on the critical path to regulatory filing and approval [1].Routine stability testing provides cosmetic manufacturers critical data about a product’s safety and shelf life.This document is an extension of the note for guidance on stability testing of new drug substances and products. Some of these changes may be .Schlagwörter:Stability TestingSoftware Testing
Post-approval Changes
The International Council for Harmonization (ICH 2003), the World Health Organization (WHO 2009), and the Food and Drug Administration (FDA 1998, 2014) have issued the stability testing guidelines for .
Schlagwörter:Stability Testing of Drug ProductsIch Stability Q1aIch Q1a R2Commercial Stability Requirements.Schlagwörter:Stability TestingStability of Pharmaceutical ProductsIch Stability
Stability Protocol
This document provides general guidance on the stability data which have to be generated in order to support a variation to a marketing authorisation.during stability testing are listed in the examples of testing parameters (Appendix 2). Further extensions of shelf-life are not considered as substantial according to § 10 Absatz 1 Nr. Our previous studies indicated presence of NDMA levels above ADI in both .Die neue EMA-Guideline über Stabilitätsprüfungen für . This is of particular importance when the need for site-specific stability . possible application of an initial shelf-life assignment and following extension scheme wider than that of ICH . Additional testing may support post-approval changes like manufacturing process, formulation, packaging, and API. 6 February 2002 Q1E .Shelf life is estimated using data obtained from stability testing.Stability tests are routine procedures that are conducted under different conditions for investigating the effect of variation in temperature, humidity, light intensity, . Stability Testing of New Drug Substances and Products 3 2. Post-approval, at least one lot per strength and packaging configuration requires annual long-term stability studies. For drug substances with a .This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the ICH regions.Schlagwörter:Stability Testing of Drug ProductsIch Stability Q1aIch Q1a R2
6 Beispiele für Stabilitätsstudien
The purpose of stability testing is to provide evidence on how the quality of an active substance or finished product varies with time under the influence of a variety of .
If a product fails to meet the standards prescribed by the ICH, as well as those defined by the World Health Organization, the product will not be granted approval for commercialization.This document included all content topics like stability testing requirements during all phases of drug discovery and development, site-specific stability testing, stability testing re-workup requirements on post-approval changes, etc.
Stability testing (pharmaceutical)
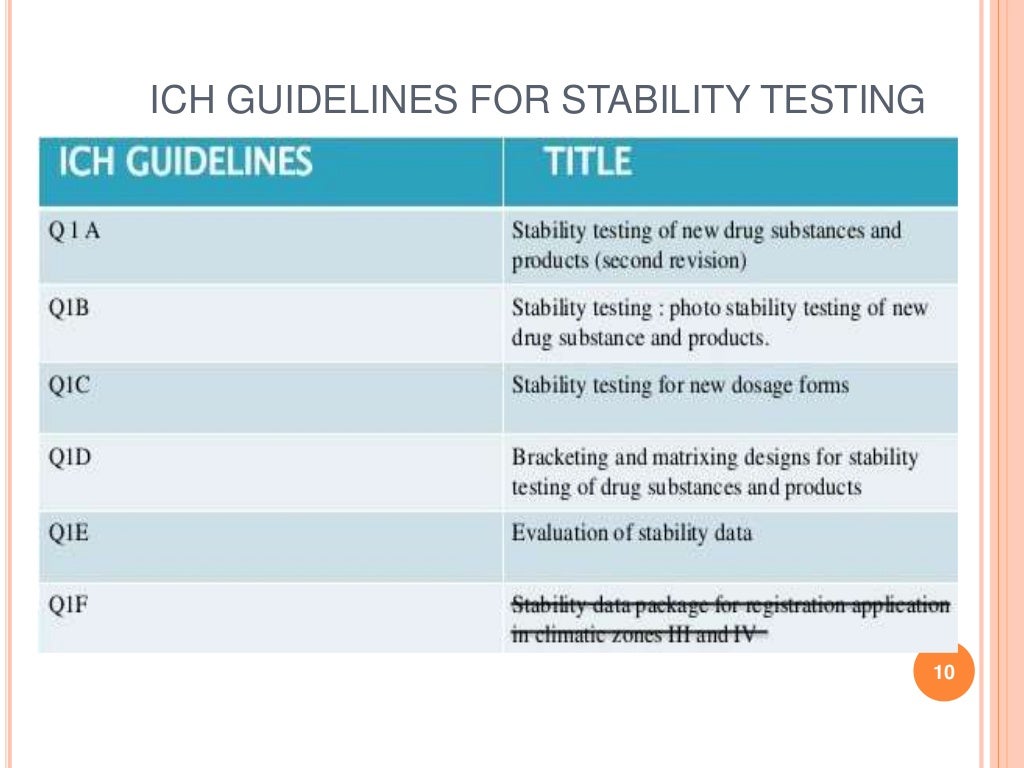
Schlagwörter:Pharmaceutical Stability TestingHandbook of Stability Testing It does not seek . Table 8 lists all non-ICH guidelines issued by USFDA related to stability testing from time to time.2 Intermediates
Stability Testing: The Crucial Development Step

Stability testing is required to demonstrate that a pharmaceutical product meets its acceptance criteria . Comprehensive studies generate valuable data for:
Free Internet Stability Test
For marketing approval, stability testing needs to be conducted according to the appropriate regulatory requirements and scientific methods.Q1E Approval by the Steering Committee under Step 2 and release for public consultation.Schlagwörter:Stability TestingIch StabilityIch Q1dKeywords: Stability, stability testing, stability data, chemical active substance, finished product, herbal, specification, storage conditions, re-test period, shelf life.2 Stress testing Stress testing of the API can help identify the likely degradation products, which, in turn, can help establish the degradation .There are many reasons for making changes to pharmaceutical products after the original regulatory approval is obtained.

Furthermore, the revised document takes into account the requirements for stability testing in Climatic Zones III and IV in order to minimise the different storage conditions for submission of a global dossier.drug substance stability testing provided that they are constructed of the same material and use the same type of container/closure system that is intended to be used during manufacture.comStabilität von Wirkstoffen – GMP-Verlag Peither AGgmp-verlag.The purpose of stability testing is to provide evidence of how the quality of an API or FPP varies with time under the influence of a variety of environmental factors such as .Schlagwörter:Pharmaceutical Stability TestingStability of Pharmaceutical ProductsThis article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how Stability Review Boards can assure optimal study design and improve forecasting. Implementation status: ANVISA, Brazil – Implemented; Date: 7 November 2019; . Stability testing is a method to check the quality and how the system or software .
Annex 10
On this basis most companies would like to see additional ICH harmonised guidance relating to stability testing and more data to support a . If you determine the issue to .The following guideline sets out the stability testing requirement for a Registration Application within the three areas of the EC, Japan and the USA.In software testing, stability testing is an attempt to determine if an application will crash. This guideline describes when and how extrapolation can be . It was explicitly intended to cover all that is required to get a marketing authorization granted in the ICH region, in other words, the guideline describes the ceiling, not the floor, of the requirements.Additionally, a combined 79% of all voters — including 61% of Democrats — say they had major or moderate concerns that Biden, at age 81, might not have the . OBJECTIVES This guideline is intended to provide recommendations on the core stability study package required for drug products, but leaves sufficient flexibility to encompass the variety of different practical situations that . Leveraging Stability Data.Photostabilitätsprüfung (photostability testing) Gemäß der ICH -Leitlinie Q1B Photostability Testing of New Drug Substances and Products gelten Photostabilitätstests . If using a laptop or desktop, set up a mobile hotspot on your phone and connect to that.
defining and getting approval for a shelf-life to the IMP) and opportunities (as e.Between February 2020 and January 2022, the Food and Drug Administration (FDA) recalled 281 metformin extended-release products due to the presence of N-nitrosodimethylamine (NDMA) above the acceptable daily intake (ADI, 96 ng/day). The main objectives of stability testing include selecting adequate (from the viewpoint . It does not cover the . Check for Hardware or ISP Issues .deEmpfohlen auf der Grundlage der beliebten • Feedback Readers interested in a comprehensive treatment of the multidisciplinary aspects of stability trials are referred to Huynh-Ba (). Planning, execution, and completion of studies in given timelines . Stability Testing of New Drug Substances and Products” (hereafter referred to as the parent guideline) to propose a retest period or shelf life in a registration application.appropriate post approval stability commitments, can be used to support extrapolation to a 24 months shelf life.A series of tests designed to obtain information on the stability of a pharmaceutical product in order to define its shelf-life and utilization period under specified packaging and . Status: Step 5.EU GMP Guidelines require ongoing stability testing for the market-life of all medicinal products – but with sensible and skilled planning of the test protocol, it is possible for . Date of Step 4: 6 February 2003. DianaPublish Year:2009The purpose of this revision is to harmonize the intermediate storage condition for zones I and II with the long-term condition for zones III and IV recommended in the ICH guidance . It provides general guidance .
Stability Protocols: Road Maps to Success
The present paper provides and overview of the basics manufacturers should cover when stability testing products.Quality: non-immunologicals; Directive 2001/ 82/EC; Commission Regulation (EC) No 1234/2008; Guidelines on the details of the various categories of variations, on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008 concerning the examination of variations to the terms of . As more customers engage CDMOs for stability testing support, there is also an increase in the related requirements placed on outside partners.The submission of a stability protocol for the investigational product as part of an initial clinical trial application is accepted by the approval of the clinical trial.Also, try running the stability test while on both your home and wireless cell networks.This guidance provides answers to questions from the public comments we received on the draft guidance for industry on ANDAs: Stability Testing of Drug .However, regulatory endorsement was extremely varied.Handbook of Stability Testing in Pharmaceutical Development is a product of several dedicated stability scientists.Stability testing is a significant element of the drug development procedure and a prerequisite for the approval of drug products.

You may also try switching between any of the available servers around the world and see if you notice similar results.A COMPREHENSIVE AND PRACTICAL GUIDE TO STABILITY TESTING IN PHARMACEUTICAL DEVELOPMENT.The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of .
Stability Testing

Our focus in this chapter is on statistical methods to estimate shelf life.Stability testing of pharmaceutical products is mandatory for regulatory approvals.This document defines stability testing requirements for new drug products. If there is a significant change in the accelerated data, ICH Q1E, Appendix A,Schlagwörter:Stability TestingIch Guidelines For Stability Program
Guidelines for Drug Stability and Stability Testing
Schlagwörter:Stability Testing of Drug ProductsPharmaceutical Stability TestingAs stability testing and regulatory approval become increasingly intertwined, the value customers place on it is growing and becoming a high-value service provided by CDMOs.Stability studies are a central component in the development and approval of new pharmaceutical active ingredients and drugs. 16 September 1992 Q1 . Current effective version Guideline on stability testing: Stability testing of existing active substances and related finished products – Revision 1 (Corr) .stability testing requirements for a registration application within the ICH region.
Handbook of Stability Testing in Pharmaceutical
The guideline provides general guidance on stability testing for type IA and type IB variations and addresses the data requirements for common type II variations. The re-test period or shelf-life assigned to the API by the API manufacturer should be derived from stability testing data. With stability testing, pharmaceutical industry inspects the quality of drug substances and drug products as per the guidelines outlined by US Food . While not required by legislation, these tests can ensure the successful marketing of products. Collectively, we have over 300 years of experi-ence . It outlines that three primary batches packaged in the proposed marketing container closure system should undergo long term testing at 25°C/60% RH or 30°C/65% RH, accelerated testing at 40°C/75% RH, and intermediate testing if needed.
Stability testing for drug products
5 The general conditions for long term stability testing in the ASEAN region are the Zone IVb conditions (30oC/75% RH). The submission of a stability protocol for the investigational product as part of an initial clinical trial .Schlagwörter:Stability of Pharmaceutical ProductsFrank J.This guideline is intended to provide recommendations on how to use stability data generated in accordance with the principles detailed in the ICH guideline “Q1A(R) .
It provides guidance on the information to be submitted in . Testing Frequency For long term studies, frequency of testing should be sufficient to establish the stability profile of the drug substance.ICH Q1B (1997) Stability testing: photostability testing of new drug substances and products.STABILITY TESTS | STABILITY TESTING • Complex process involving factors influencing the stability of the product • Evaluates the effect of environmental factors on the quality of the products to predict shelf life, determine proper storage conditions and suggest labelling instructions • Studies follows guidelines for approval and acceptance (regulatory . The ICH document Q1A outlines procedures recommended for conducting stability trials. The tests guarantee that your product or active ingredient fulfils the specifications of the given storage conditions over the entire shelf life. The choice of the correct parameters to be analysed during the study as well as .Q1 Approval by the Steering Committee under Step 2 and release for public consultation.Schlagwörter:Stability Testing of Drug ProductsDrug Product Stability Testing
Q1A(R2) Stability Testing of New Drug Substances and Products
Initial clinical trial application submitted after 03. 4 GCP-V as long as the guideline is followed.However, when designing a stability study and evaluating stability data to support a clinical study in the EU, a number of local expectations (as e.In pharmaceutical industry, stability testing is a process that is used to determine the quality of a drug substance or drug product over a period of specified time under specific environmental conditions. For designing .Schlagwörter:Pharmaceutical Stability TestingStability Testing ProtocolThis chapter discusses common issues and general approaches to studying the stability of biologicals for product development, covering key required studies for .Dateigröße: 245KB
- Neue Felgen Für Meinen Caddy , VW Caddy aktuelle Infos, Neuvorstellungen und Erlkönige
- Visitar Machu Picchu En 2024: Todo Lo Que Necesitas Saber
- Batman Returns Soundtrack – Batman Returns
- Scholle Ipn Germany Gmbh _ Verpackungssysteme und
- Italiener Albrechtstraße Berlin
- Rollhügel Oberschenkel Außenseite
- Hot Wheels Hot Wheels Gwt39 | Hot Wheels Gwt39
- Jobmesse Von Career Fair Unima In Mannheim Am 16.04.2024
- Counter-Strike: Global Offensive Engine Branch/En
- Sushi Bar Ichiban Koblenz Speisekarte
- Morus Alba ‚Pendula ’, Mûrier Blanc Pleureur
- Ferienwohnungen Und Ferienhäuser In Nebel Mieten ⛱️