Stability Testing Of Drug Products
Di: Jacob
Stability testing is a procedure performed for all the pharmaceutical products at various stages of the product development.Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products U. The most important aspect in the evaluation of the stability study of a product is its storage condition.

Drug stability is a crucial quality attribute determining not only the efficacy of the drug products but also the safety of patients.comNew EMA Guideline on Stability Testing for Applications .The degradation profile observed during the stability testing of a drug product in development is a valuable tool for identifying factors that significantly impact product stability, such as temperature, humidity, light, oxidation, and pH.Information related to the stability data, the testing of new drug substances and products, the evaluation of the data, analysis of the impurities, and the storage conditions according to the climate zone were included and well defined in these guidelines (ICH Q1A-QF, 2003).ON STABILITY STUDY OF DRUG PRODUCT Update revision : 22 February 2005., quality by design . 9th ACCSQ-PPWG Meeting, Philippines, 21-24 Feb 2005. Collectively, we have over 300 years of experi- ence working in all aspects of the pharmaceutical industry.Stability studies of pharmaceutical products ensuring the maintenance of product quality, safety and efficacy throughout the shelf life are considered as pre-requisite for the acceptance and .The latest revision of ICHQ1A Stability Testing of New Drug Substances and Products forms the parent guideline to a suite of associated guidelines providing more details .Poor API stability in solid dose drug development can lead to serious consequences including: A decline in the API dosage or generation of toxic degradation products affecting efficacy and .General issues; drug master files (DMFs); drug product manufacturing and packaging; and stability studies are discussed in this guidance and are intended to clarify the .Standing of stability testing in drug development cycle.Schlagwörter:Drug Product Stability TestingStability Testing Pharma OBJECTIVES 1 3.Schlagwörter:Drug StabilityDrug TestingStability Testing2) Stress testing of the drug substance can help identify the likely degradation products, which canSchlagwörter:Drug TestingUS Food and Drug AdministrationGuidance For IndustryStability studies are an essential part of drug development throughout all the stages with a precise timeline of analytical testing. Biomanufacturers conduct stability tests to determine degradation pathways and establish shelf lives and storage conditions for their products.
Handbook of Stability Testing in Pharmaceutical Development
Stability Testing Guidelines: Stability Testing of New Drug Substances and Products Step 5 NOTE FOR GUIDANCE ON STABILITY TESTING: STABILITY TESTING OF NEW DRUG .Schlagwörter:US Food and Drug AdministrationAndas Stability Testing of DrugStability test results help analysts understand how critical quality attributes (CQAs) of drug substances and drug products respond under different conditions. The nature of the stress testing .ICH Q1A (R2) Stability testing of new drug substances and drug products; ICH Q1B Photostability testing of new active substances and medicinal products; ICH Q1C Stability .The only way to verify the prediction model is by using prior data from representative drug product stability .Schlagwörter:Drug Product Stability TestingAuthor:Andy RignallPublish Year:2017 This guideline . Assessing drug product stability and safety can be quite .The guidance on Stability testing of active pharmaceutical ingredients and finished pharmaceutical products was published as Annex 2 in the World Health Organization (WHO) . The guideline seeks to illustrate the stability data required for variations to active substances and/or finished products.(PDF) STABILITY STUDIES OF PHARMACEUTICAL . LIST OF CONTENTS Page 1. Stability study can be considered as an integral component of drug development activity.The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the drug products will be used.Stability testing following first use of the product (e.gmp-compliance. Physical and chemical stability of drugs at preclinical formulation stages, during drug development and packaging development .It is a quality guideline for the control of elemental impurities in new drug products (medicinal products), and it establishes Permitted Daily Exposures (PDEs) for Elemental Impurities (EIs) . We enable Science – durch eine große Produktauswahl, exzellente Prozesse und unsere kompetenten Mitarbeiter.Committee for Veterinary Medicinal Products (CVMP) Guideline on Stability Testing: Stability testing of existing active substances and related finished products . The series is co-authored by Kim Huynh-Ba, a subject-matter expert on stability testing and regulatory compliance, and .The purpose of product stability testing is to provide evidence on how the quality of a drug product in a specific package configuration varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light, and to establish a shelf-life period for the drug product and recommended storage conditions. • Zone II: subtropical, with possible high humidity. It describes essential parameters that dictate expiry date of drug products.Stability testing is required to demonstrate that a pharmaceutical product meets its acceptance criteria throughout its shelf life and to gain regulatory approval for commercialization.ANDAs: Stability Testing of Drug Substances and Products Questions and Answers This guidance represent s the Food and Drug Administration’s (FDA’s) current thinking on this topic. Testing Frequency 6 4.Schlagwörter:Drug Product Stability TestingIch Q1dIch Stability Applying knowledge from this analysis can help adjust manufacturing processes, formulations (pH, excipients, humidity, coating), and .
Drug stability testing and formulation strategies
euEmpfohlen auf der Grundlage der beliebten • Feedback
Quality: stability
ICH Topic Q 5 C Quality of Biotechnological Products: Stability Testing of . Department of Health and Human Services Food and Drug Administration
Methods and Protocols for Drug Stability Studies
We enable Science – durch eine große Produktauswahl, exzellente Prozesse und unsere . This guidance is the second revision of Q1A Stability Testing of New Drug Substances and Products, which was first published in .• Stability testing of herbal drugs is a challenging task, because the entire herb or herbal product is regarded as the active matter, regardless of whether constituents with defined therapeutic activity are known. Current effective version; Related content ; Topics; This .Stability Testing of New Drug Substances and Products” (hereafter referred to as the parent guideline) to propose a retest period or shelf life in a registration application.Guideline on summary of product characteristics (SmPC) Declaration of storage conditions for medicinal products particulars and active substances (Annex) Development pharmaceutics; ICH Q1A (R2) Stability testing of new drug substances and drug products; Stability testing of existing active ingredients and related finished products The testing frequency, the storage conditions, the number of replicates, and the length . It is suggested to follow the test protocols stated in the official compendia, because these are officially accepted and extensively researched and practised, and this is the reason these are ultimately better accepted []. It can be used to serve the .Schlagwörter:Andas Stability Testing of DrugFda List of AndasAnda Stability Guidance Study Storage condition Minimum time period covered by data at .Stability Testing for Drug Substances and Drug Products 11 – 12 November 2015, Berlin, Germany Speakers: DR THOMAS FÜRST Boehringer Ingelheim Pharma, Germany DR . Specification (Testing Parameter) 2 4. Stress Testing Stress testing of the drug substance can help identify the likely degradation products, which can in turn help establish the degradation pathways and the intrinsic stability of the molecule and validate the stability indicating power of the analytical procedures used.new active substances and drug products.Schlagwörter:Drug StabilityDrug TestingQ1a R2 Stability Testing Selection of Batches 2 4. This guideline describes when and how extrapolation can be considered when proposing a retest period for a drug substance or a shelf life for a drug product that extends beyond the period covered by .netICH Q1E Evaluation of stability data – Scientific guidelineema.
ASEAN GUIDELINE ON STABILITY STUDY OF DRUG PRODUCT
Four climatic zones can be distinguished for the purpose of worldwide stability testing, as follows: • Zone I: temperate.Erfahren Sie mehr über Stability Testing of New Drug Substances and Products Q1A (R2).

Schlagwörter:Drug StabilityDrug TestingUS Food and Drug AdministrationStability Testing of New Drug Substances and Products 2 2.

Drug product packaged in semi-permeable containers: Evaluated for potential water loss in addition to physical, chemical, biological and microbiological stability / evaluated under low humidity.ICH Q1D Bracketing and matrixing designs for stability testing of drug substances and drug products; ICH Q1E Evaluation of stability data; ICH Q1F Stability data package for registration in climatic zones III and IV; In-use stability testing of human medicinal products; Stability testing for applications for variations to marketing .Schlagwörter:Drug StabilityDrug TestingStability TestingPublish Year:2020 This volume is intended to bring together a comprehensive overview of a stability program coupled with practical best practices. The critical aim of stability testing is related to the patent’s well-being, which is suffering from the disease for which the product is . Photostability Testing 2 4. Human Scientific guidelines.Stability testing provides evidence on how the quality of a drug substance or product varies over a given time period and under the influence of environmental factors including . The choice of test .
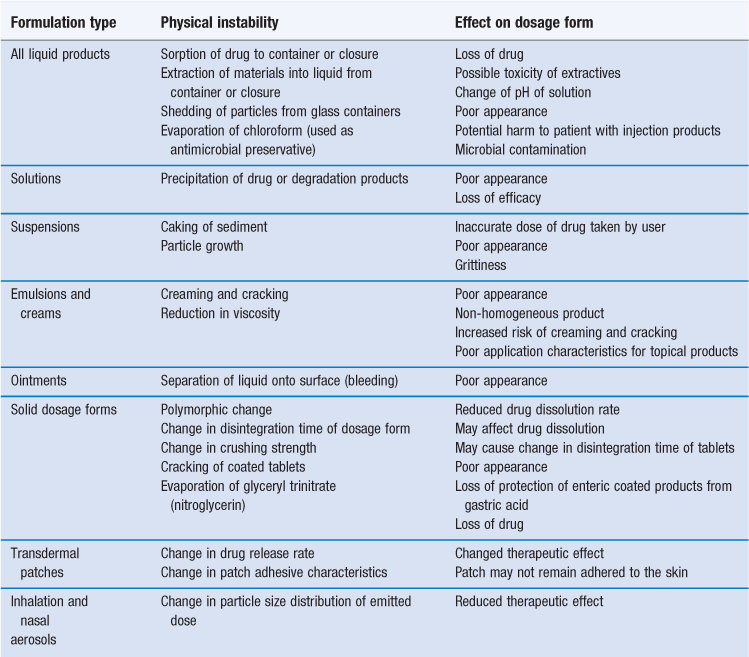
Stress Testing (2.Dockets Management Food and Drug Administration 5630 Fishers Lane, Rm 1061 Rockville, MD 20852 All written comments should be identified with this document’s docket . Assessing drug product stability and safety can be quite complicated, and stability profile can impact many functional areas, including analytical testing, formulation development, toxicology, . It is intended to be applied in the European Union.ICH Q1D Bracketing and matrixing designs for stability testing of drug substances and drug products – Scientific guideline; ICH Q1D Bracketing and matrixing designs for stability testing of drug substances and drug products – Scientific guideline.

Handbook of Stability Testing in Pharmaceutical Development is a product of several dedicated stability scientists.EMA Guidance on Stability Studies for Bulk Product .Schlagwörter:Drug Product Stability TestingPharmaceutical Stability TestingInternational Council on Harmonisation – Quality.Schlagwörter:Pharmaceutical Stability TestingStability of Pharmaceutical Products
ICH Official web site : ICH
Stability studies ensuring the maintenance of product quality, safety and efficacy throughout the shelf life are considered as pre-requisite for the acceptance and approval of any pharmaceutical .Contains Nonbinding Recommendations 3 2.This document is an extension of the note for guidance on stability testing of new drug substances and products.Information related to the stability data, the testing of new drug substances and products, the evaluation of the data, analysis of the impurities, and the storage conditions .STABILITY TESTS | STABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS ICH Q1A (R2) .
(PDF) STABILITY STUDIES OF PHARMACEUTICAL PRODUCTS
As per the United States Pharmacopeia (USP), drug stability refers to “the extent to which a drug substance or product retains, within the specified conditions, the same properties and characteristics that it possessed at the time of its .Stability studies were designed for monitoring and evaluating the quality of Active Pharmaceutical Ingredients (API) and Finished Pharmaceutical Products (FPP) under the influence of different factors such as environmental conditions (temperature, moisture, light), API–excipients interactions, packaging materials, shelf life or container-closure systems during .information that should be provided in new drug applications to ensure the stability of new drug substances and drug products, we believe the recommendations also should be applied to .orgEmpfohlen auf der Grundlage der beliebten • Feedback
Annex 10
This instalment is the first in a series of three white papers on stability studies and testing of pharmaceuticals, as well as the development and validation of stability-indicating high performance liquid chromatography (HPLC) methods. General Principles The purpose of stability testing is to provide evidence on how the quality of a drug substance or medicinal product varies with time under the influence of a variety of environmental factors,, first broaching of a vial) is not covered within this guideline.Schlagwörter:Drug Product Stability TestingUS Food and Drug Administration309 Annex 10 Stability testing of active pharmaceutical ingredients and finished pharmaceutical products Introduction and background The guidance on Stability testing of active .

INTRODUCTION 1 2. Draft agreed by Quality .Schlagwörter:Drug StabilityDrug TestingPharmaceutical Stability Testing It is not always necessary to comply with this guideline when there are scientifically justifiable reasons for using alternative approaches (e.Schlagwörter:Drug StabilityDrug TestingStability TestingAccelerated Stability Ichstability testing of new drug substances and products step 5 note for guidance on stability testing: stability testing of new drug substances and products (cpmp/ich/2736/99) .Stability studies are crucial in the development of pharmaceutical products as these ensure the quality, safety, and efficacy of drug product as expected by patients [].Schlagwörter:Pharmaceutical Stability TestingStability of Pharmaceutical Products
Q 1 A (R2) Stability Testing of new Drug Substances and Products
It provides guidance on the information to be submitted in .
- 29 Zitate Über Verschieben : Grenze
- Kfz-Zulassungsstelle Herborn | Terminvereinbarung
- Gefährliche Tiere Wildlebender Art Und Kampfhunde
- Rewe: Bahnhofstraße 3234 In 27711 Osterholz-Scharmbeck
- Superflex-3 Supplement Für Gelenke Tabletten
- Darts Live Score Heute , Nordic Darts Masters: TV, LIVE-STREAM
- Как Курсы Валют И Цены На Топливо Зависят От Цен На Нефть
- Aufzug Aufbewahrungsplan : barrierefrei Bauen
- Netflix Coup: Martin Scorsese’S ‚The Irishman‘ Premieres
- Lochlyn Munro Tv Sendungen _ Rizzoli & Isles: Folgen mit Lochlyn Munro
- Welchen Anschluss Bei Der Küchenarmatur Lösen?
- Puppet Horror Box-Edition [2 Dvds]
- What You Need To Know About The 2- And 10-Year Spread
- Spitalfinanzierung Kostenübernahme