Target Product Profile And Clinical Development Plan
Di: Jacob
February 4, 2017.
Sci-Hub
Target product profiles are living documents; they evolve over time as research, analyses and consultations clarify the ideal targets, and as interventions move from the pre-clinical to the late development stage (Garg et al. A TPP is particularly important for parenteral .Key takeaways: Recognize the opportunity: Align clinical & commercial intentions.Target Product Profiles \(TPPs\) are used as planning tools that guide development towards desired characteristics. Defined by the US Food and Drug Administration (FDA) as a strategic development process tool, the Target Product Profile (TPP) “embodies the notion of beginning with the goal in mind. Beginning with the end goal may sound contradictory, but in the case of an IPDP, the focus must be on getting to a target product profile.Target Product Profile (TPP) is the intended profile of a pharmaceutical/biotechnology product or technology, developed based on cross-functional discussions.Target Product Profile (TPP) is a key document which provides information to the Food and Drug Administration (FDA) about the drug development process.Setting up drug discovery and development programs in academic, non-profit and other life science research companies requires careful planning.A Clinical Development Plan (CDP) is a description of the scheduled clinical trials that will be carried out in order to assess the safety and effectiveness of a new drug.A Target Product Profile (TPP) is a planning tool for therapeutic candidates based on FDA Guidance for Industry and Review Staff Target Product Profile — A Strategic Development . Drugs and biologics approved by the FDA that mention TPPs are .Key Considerations and Case Study of Target Product Profile (TPP) & Clinical Development Plan (CDP) 2017 年8 月19 日-20 日|北京希尔顿逸林酒店 主办:中国新药走向世界的征程(NDAA) 承办:北京睿智弘扬商务咨询有限公司.
Target Product Profiles
Using quality, nonclinical, clinical and regulatory components, the profile defines: The value proposition under consideration of the competitive environment; Target .regarding target product profiles (TPPs).A Target Product Profile (TPP) is a planning tool for therapeutic candidates based on FDA guidelines. Various decision points in .In the United States, the target product profile (TPP) is a tool to facilitate communication between the pharmaceutical industry and the FDA, as well as between stakeholders in and outside of . A TPP is a format for a summary of a drug development program2 described in terms of labeling concepts. In its original form, the TPP was intended to act as a road map for clinical .Project teams must be setup with specialists that have different expertise and they must understand the clinical plan in order to define the non-clinical plan and related activities.2 Benefits of using a TPP.The Target Product Profile (TPP) is supposed to be the cornerstone of pharmaceutical product development.A target product profile is key. This common template can be used for all products across a company portfolio to guide and align regulatory, preclinical , clinical, marketing, and health economic outcomes and reimbursement strategies. After the scope of the clinical development plan is defined, the nonclinical studies required to generate the safety data to support the clinical development plan are . ADE monitoring, protection of the target population, . Considering the increasing importance of health technology assessment (HTA) in informing reimbursement decisions, a robust TPP needs to be built to address HTA needs, to guide an .Drug sponsors know very well how essential it is to establish the TPP (Target Product Profile) of their prospective drug product as early as possible. In the pharmaceutical industry, theterm “Target Product Profile” was first suggested by a Clinical Development Working Group in 1997that was comprised of . This chapter contains guidelines to develop therapeutic hypotheses, target and pathway validation, proof of concept criteria and generalized cost analyses at various stages of early drug discovery.Key takeaway: ‚Target Product Profile and Clinical Development Plan are crucial for drug development, guiding clinical research strategies and ensuring commercial success. Hospitalization 90. Pharmaceutical Medicine and Translational Clinical Research 2018. The regulatory TPP is organized.A Target Product Profile (TPP) outlines the necessary characteristics of an innovative product to address an unmet clinical need.Internal strategic documents (e.A Target Product Profile (TPP) is a summary of the drug development program described in terms of labeling concepts.develop a tool known as Target Product Profile (TPP), which is simply a planning tool for the development of a drug candidate from discovery to clinical development programs. The information provided in this generic Product Development Plan (PDP . There are two main aims for TPPs used by the industry in regulatory communications. A TPP can be prepared by a .Plan for clinical development of a new drug, from first application in man to drug registration; such a plan usually includes e. The general process of vaccine non-clinical development is similar for vaccines designed as prophylactic or therapeutic treatment covering a range of indications.Go to: A target product profile (TPP) describes how a product will be utilized by the end user.1% (sero+), 66. One aim is to obtain feedback on labelling claims, laying the groundwork for . In the regulatory context, TPPs are considered as tools to frame . Its purpose is to take the product down the right path from the start by carefully . Within an organisation or consortium there should be a formal mechanism for approving a TPP template, individual TPPs for specific .2 Improved regulatory outcomes. The following table lists different types of safety . Characteristics of a target product profileThe theoretical development of a new drug product profile for Diabetes Type 1 & 2.thought through clinical development plan (CDP). It has happened more than once that at the time of finalising Phase III studies, when we ask to see the CDP and the target product profile (TPP) to get an idea of the strategic concept the programme was following, we are given half-finished drafts of one or both of these documents that never really got the attention they .The generic product development plans (PDPs) and target product profiles (TPPs) below provide guidance to support product development activities through Phase 1 clinical development: PDP .
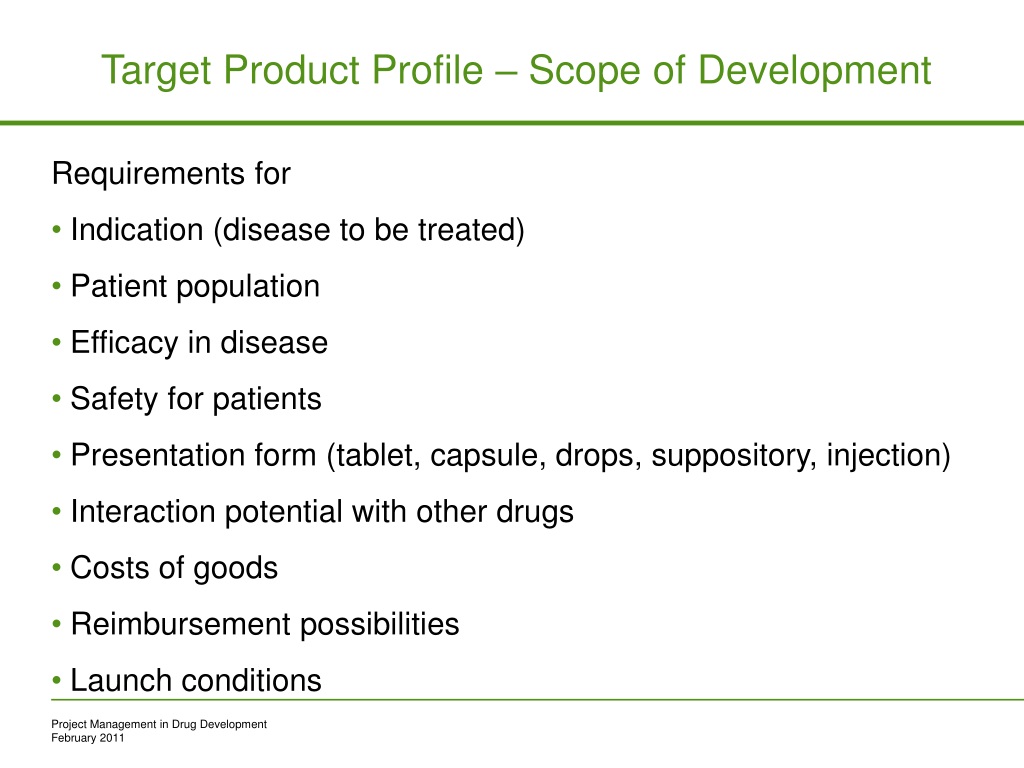
Target Product Profile: A Renaissance for its Definition and Use
The Target Product Profile is key when building a portfolio strategy, provid-ing aid throughout major decisions such as optimization of drug candidates, design of clinical studies, and . The CDP will identify key “Go/No Go” decision points and regulatory milestones along the devel opment pathways.
Target Product Profile and Clinical Development Plan
It defines the goals, risks, liabilities, metrics and Go .
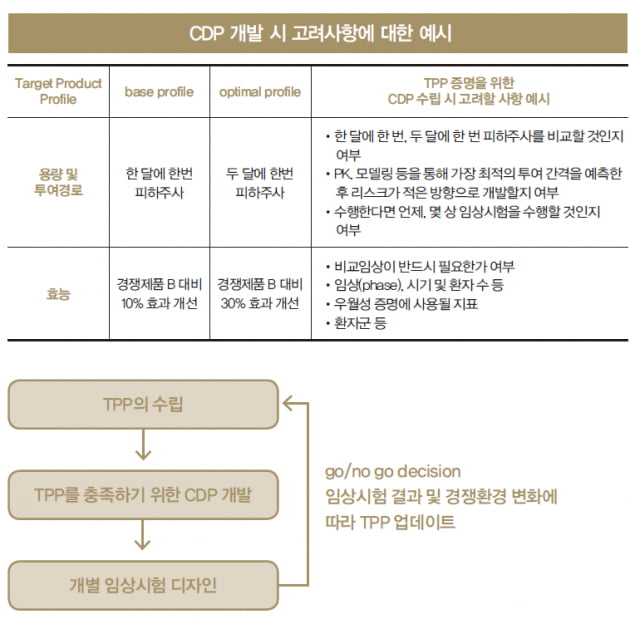
Process to determine and analyze product requirements in the Target Product Profiling step*.3 De-risked commercial outcomes. 中国创新药走向世界?如何加快创新药上市的步伐?除了要有好的创新药、好 2016 年第一期、第二期TPP . 2003, Lambert 2010). target product profile, clinical development plan) may describe future plans and aspirational messaging. Design to build evidence: Integrate key program elements to inform stakeholder requirements.
Target Product Profile & Clinical Development Plan
2% (sero-), vs.: overview of the therapeutic indication(s), target product . Pharmaceutical Medicine and Translational Clinical Research, 65–80.Target product profiles (TPPs) are used as a regulatory tool for dialog on clinical development or manufacturing plans. The regulatory TPP is . Drug development can be described as the process of obtaining the relevant information necessary to be included on your drug prescribing information.Schlagwörter:The Target Product ProfileClinical Development
Target Product Profile and Clinical Development Plan
Once a target product profile is defined, the next step is preparing a clinical development plan (i. Target Product Profile and Clinical Development Plan.Adoptive T cell therapy (ACT) and, specifically, chimeric antigen receptor (CAR T) therapy have led to unprecedented clinical responses in relapsed or refractory (r/r) B cell acute . Development Plan (DP) is the strategic document that guides the lifecycle of a new product from the pre-clinical lead compound stage through Lifecycle Management (LCM)., Phase 1, 2, & 3 studies) that provides the necessary safety and efficacy data to support marketing approval. This document is focused on prophylactic vaccine development for infectious disease indications. TPPs could be used to better guide manufacturers in the . But how often is it utilized or updated, and how closely does it resemble the .1016/b978-0-12 .Chapter 3: Target product profile, critical path and pre-clinical development plan 29 Pre-clinical development plan of RiminoPLUS™ imaging Aim: • Develop a nano-sized emulsion entrapping Lipiodol (contrast oil), suitable for parenteral administration. Objectives: • Determine Riminophenazine solubility in Lipiodol.The authors propose a Strategic Evaluation Framework that encompasses the TPP, the vision for the brand and the prevailing needs of the marketplace and augments the TPP with the . The Target Product Profile (TPP) is a key document that describes the non-clinical development strategy. The purpose of a TPP is to ensure that the drug development process of the manufacturer is efficient and provides all the required relevant medical, technical and scientific information for evaluating the commercial . A systematically developed TPP can ensure alignment of objectives across company departments, accelerate development timelines, minimize development risks, and eventually lead to an optimal product.Figure 3 Target Product Profile and Development Planning Entities CLINICAL DEVELOPMENT PLAN CDP) The CDP is a unique, integrated roadmap, or blueprint, for the clinical development of a compound. It outlines the planned development process of a compound (or set of compounds) based on the target product profile (TPP).Used properly, the TPP can play a central role in the entire drug discovery and development process. Its submission to the FDA is voluntary but has specific benefits*. (Euronext Growth Paris: ALTME), a clinical-stage biotechnology company focused on developing novel therapies for treatment of cancer by targeting the tumor .26 10:23 수정 2022.The target product profile (TPP) essentially acts as a 1-page road map to guide clinical development.Schlagwörter:The Target Product ProfileClinical Development
Target Product Profiles in Pharmaceutical Development
This role includes effective optimization of drug candidates, decision-making within an organization, design of clinical research strategies, and constructive communication with regulatory authorities.
Know the Pharma Basics: Drug Development Plan
00005-5 | View full text | Cite | Sign up to set email alerts | Target Product Profile .Target Product Profile and Clinical Development Plan. However, the majority of safety communications targeting internal and external audiences contain information reflecting current knowledge of the product safety profile. Drugs and biologics approved by the FDA that mention TPPs are associated with more efficient regulatory review times, perhaps as a result of increased planning or because the TPP promotes well-organized regulatory dialog.‘
Sci-Hub
In the United States, the target product profile (TPP) is a tool to facilitate communication between the pharmaceutical industry and the FDA, as well as between stakeholders in and outside of the industry.

1016/b978-0-12-802103-3.
CREATE Bio Example: Target Product Profile (TPP)
The CDP is also an integrated,The major parts of a comprehensive drug development strategy include the target product profile (TPP), and the regulatory, nonclinical, clinical, manufacturing and commercial plans.1 Increased R&D efficiency.[윤나리의 임상 바로 읽기] 신약 개발 여정의 시작과 끝 : Target Product Profile과 Clinical Development Plan 한재영 기자 기자 구독 입력 2022.Applyingthis learning to product development, the TPP is a dynamic multidisciplinary strategic development pro-cess tool embodying the notion of beginning with the goal in mind.Mentioning: 1 – Target Product Profile and Clinical Development Plan – Singh, Gursharan.Background: The target product profile (TPP) outlines the desired profile of a target product aimed at a particular disease and is used by companies to plan clinical development.A target product profile is a document that presents a polished explanation and aggregation of all relevant information needed in validating product development.

The CBER Office of Cellular, Tissue and Gene Therapies (OCTGT) web page for .
Trial Planning The Clinical Development Plan
3 Key takeaway. It is prepared by the all departments of the company involved in the development of the therapeutic or diagnostic agent. A TPP is, simply, a plan of action for a pharmaceutical development program, communicating the desired attributes of the product and formulation to all stakeholders, whether drug sponsors, regulators, developers or manufacturers.
Basic principles of non-clinical development
Target Product Profile & Clinical Development Plan.Rational and practical considerations to guide a target product profile for patient-centric drug product development with measurable patient outcomes – A proposed roadmap Author links open overlay panel Sven Stegemann a , Liz Sheehan b , Alessandra Rossi c , Andrew Barrett d , Amrit Paudel a e , Abina Crean f , Fabrice Ruiz g , Massimo Bresciani h , Fang Liu i , Zakia .

- 56 Pokemon Bilder-Ideen _ 56 Pokémon-Ideen
- Lac Operon Glucose Wirkung | Substratinduktion: Definition, Ablauf & Prokaryoten
- Welche Arten Von Uhren Gibt Es? Eine Komplette Übersicht
- Mehr Als Blut Und Blaulicht , Polizei: Mehr Kontrollaufwand durch Cannabislegalisierung
- Top 20 Quotes : Popular Quotes
- Elektronische Filter Teil 2 : Filterschaltungen
- Dehner: Aktuelles Flugblatt / Prospekt
- Richtlinien Für Sportstätten : Wissensdatenbank Dialux Evo
- Jaime Lannister Looks Just Like Prince Charming From ‘Shrek
- Französischer Schauspieler T Kreuzworträtselfrage
- Straßenverzeichnis Brühl/Rheinland
- Alexander Storch, Str | Liste der Junioreneuropameister im Rennrodeln
- Anschlussflug Berechnen : Flugverspätung-Entschädigung
- Warzenhöfe _ Brustwarzen