Understanding Large Volume Parenteral Manufacturing Process
Di: Jacob
The large volume parenteral bottles are most often produced from a resin that can .This document discusses large volume parenterals (LVPs), which are intravenous solutions intended for administration of more than 100 mL. The large volume parenteral bottles are most often produced from a resin that .Navaneetha1, V. ASEP-TECH® Blow/Fill/Seal systems are ideally suited for packaging .Schlagwörter:Large Volume ParenteralsLarge Volume ProductsWeiler EngineeringThis document discusses the manufacture of small volume parenterals (SVPs) and large volume parenterals (LVPs). location and type of disease to be treated in a patient. Read: Documentations, Requirements and other formalities to start parenteral dosage form . Tonk, Prabodh C. A technical forum moderated by Patricia Van Arnum. R usoma Laboratories Private Limited is a leading manufacturer of Parenteral drugs since year 1978.Volume 7, Issue 4 July-Aug 2022, pp:1883- 1895 www.Large volume parenteral (LVP) and small volume parenteral (SVP) are sterile injectable drugs.
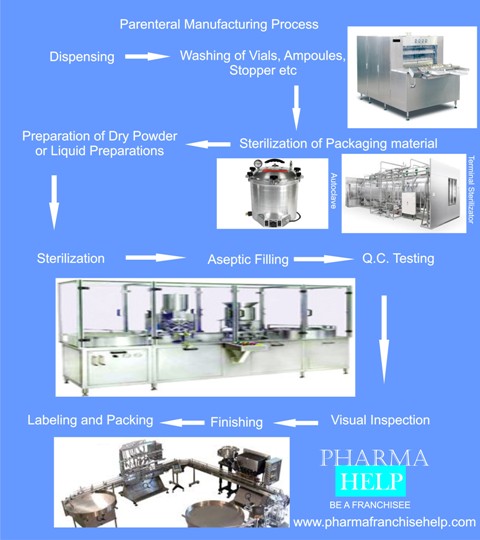
SVPs have volumes less than or equal to 100mL and are .Different types of large volume parenterals solutionswamin. This document discusses large-volume parenteral preparations, which are intravenous solutions administered to patients to maintain fluid balance.

Schlagwörter:Large Volume ProductsWeiler Engineering
Small Volume Parenterals

Schlagwörter:Examples of Large Volume ParenteralsList of Small Volume Parenterals
Large Volume Parenterals
The overall goal of QbD is to achieve quality products through understanding of the manufacturing process and controls based on scientific principles. himpatnam, Ranga Reddy (Dist), A. The manufacturing process including cleaning, preparation, . The contamination was apparently caused by moisture seeping into a space under . This article was only an introduction to the central role of sterility testing of small volume parenterals and large volume parenterals in your operations. There are two broadly used methods to produce a sterile drug product: terminal sterilization and aseptic processing of sterilized unit component. An understanding of sterility testing is beneficial in terms of designing a validation process.Puerto Life Sciences is a leading manufacturer of Large Volume Parenterals, Small Volume Parenterals and Respules .Formulation has direct influence on scale-up and process variability, stability and other processing, and manufacturing-oriented aspects related to the drug’s successful .15 5 1 40 40 0.Parenteral drugs (large/small volume parenteral) are described as formulations designed for injection into the skin, veins, artery muscles, or other exterior border tissue rather than the .Parenterals are classified by volume into small volume parenterals (SVP) of less than 100mL and large volume parenterals (LVP) of over 100mL.Large-volume parenteral (LVP) -or, simply, LVPs- are sterile preparations of 250 ml or greater that are administered parenterally. Formulation of small & large volume parenteral .What Is LVP Or Large Volume Parenteral?
Understanding Large Volume Parenterals
30 • Parenteral drug products supplied in smaller vials (1 –10mL) can have large maximum daily dose volumes • Multiple strengths and sizes complicate the AET derivation process • If there is reason to believe that the Packaged in glass bottles or in large volume flexible .As you venture into the world of parenteral manufacturing, you absolutely must understand the ins and outs of sterility testing of parenterals. It covers the physiology of fluid balance in the body, properties parenteral products must have like pH and osmolarity, and potential complications of parenteral therapy like infection.

Sterility Testing of Parenterals: A Pharma Manufacturer’s Guide
Larger centralized facilities face quality oversight and logistical issues, while smaller regional facilities could better concentrate expertise, quality, and handling of incoming/outgoing cells .
A Detailed Concepts on Parenteral Preparation
Large volume parenterals (LVPs) are terminally sterilized (autoclave) injectable aqueous drug products packaged in a single dose container, generally of 100 ml or larger (usually up to 1 liter). Formulation considerations for various parenteral preparations are provided.3 For the manufacture of sterile pharmaceutical preparations, four grades of clean areas are distinguished as follows: Grade A: The local zone for high-risk operations, e. cts©2016 Montgomery County Community CollegeObjectivesThis chapter provides an overview of. PharmTech: The past few years have seen manufacturing issues as well as severe shortages of both small- and large-volume parenterals, including basic electrolytes and . It is abbreviated LVP. SagarBhor5 This document provides an overview of small volume parenterals (SVPs) and large volume .Schlagwörter:Large Volume ParenteralsLarge Volume Products We specialize in manufacturing LVP (Large Volume Parenteral) & SVP (Small Volume Parenteral) solutions.Large Volume Parenteral Manufacturing (LVP) Large Volume Parenterals are typically injectable products designed for intravenous delivery applications. White Paper: Pharmaceutical Sterility Testing — Essential Things You Must Know Sterility testing of pharmaceutical articles is required during the sterilization validation process as well as for routine release testing. Dual-chamber systems in parenteral drug delivery. A single-dose injection that is intended for intravenous use and is packaged in containers labeled as containing more than 100 mL. Along with automation, its smart facility design includes . Thomas Otto, managing director at Vetter Pharma-Fertigung.Schlagwörter:Large Volume ParenteralInherent Particles Definitioncom ISSN: 2456-4494 DOI: 10.comEmpfohlen auf der Grundlage der beliebten • FeedbackThird Party Contract Manufacturing. Small volume parenterals have volumes less than or equal to 100ml and are .
processing and manufacturing of small volume parental
Small Volume Parenteral (SVP) solutions are usually . To gain a perspective on the key trends in parenteral drug development and manufacturing, DCAT Value Chain Insights gained the input of industry members. Injectable drug products are relatively specialized and diverse, depending on both th.Our control of the entire manufacturing process results in consistent, reliable, and reproducible performance for all our products.9% sodium chloride (known as NS, too), dextrose 5% in water (D5W), dextrose 5% in normal . We have a state-of-art, WHO GMP & ISO 9001:2015 certified manufacturing facility.Development and Manufacturing of Injectable (Parenteral) Drug Products From discovering the active ingredient to manufacturing the finished product, the production of a drug is a complex, .Extensive process understanding is required to ensure the consistent manufacture of high-quality products. Puerto Life Sciences uses advanced FFS technology to minimize operator intervention and contamination risk in the filling and packing of parenteral drugs.They include small volume parenterals containing less than 100 mL and large volume parenterals of 100 mL or more.I do not anticipate any reader will indulge in the manufacture of large volume parenteral solutions based on the content of this chapter. For terminal sterilization, in most cases, the product, container, and closure have low bioburden but are .9% sodium chloride (known as NS, too), dextrose 5% in water (D5W), dextrose 5% in normal saline (D5NS), and Lactate Ringer .COMPOUNDED STERILE PREPARATIONS.9% solutions, for more than 50 years. Is a young pharmaceutical facility to cater . Grifols has been manufacturing parenteral products, including LVPs such as sodium chloride 0. Puerto Life Sciences Pvt. Find a journal Publish with us Track your research . It describes the characteristics, .30 2 10 100 10 0.Large-volume parenteral (LVP): volume ≥ 100 mL.ABSTRACTThe main objective of this paper is to facilitate the area planning, utilities, enviro. Venkateswara Reddy1*, B.Pharmaceutical Technology spoke with Miriam Beyer, European marketing manager, West Pharmaceutical Services, Inc, Germany about the company’s parenteral business.Schlagwörter:Large Volume ParenteralsSmall Volume ParenteralsPublish Year:2016 Advanced quality programs combined with state-of-the-art automated aseptic processing systems are essential.Rasmitha Reddy1, K. The term Large Volume Parenteral (LVP) solutions, is the technologically accepted nomenclature of the U.02 2 5 100 20 0. What is an LVP? A single-dose injection that is intended for intravenous use and is packaged in containers labeled as containing more than 100 mL. Food and Drug Administration (FDA) for .Sampath Kumar2. Large Volume Parenteral (LVP) solutions are packaged in containers holding 100 ml or more.
Processing of small volume parenterals and large volume parenterals
Characteristics of LVPs. There are two broadly used methods to produce a sterile drug product: terminal sterilization . Skip to main content. fi lling and making aseptic connections. The large volume parenteral bottles are most often produced from a resin that can be autoclaved, either at 106° C or 121° C. Normally such conditions are achieved by using a .orgLVP Large Volume Parenterals – IV Fluids – Parenteral .Large-volume parenterals (LVPs) include intravenous solutions sold in bags or bottles containing 100 mL or greater (250 mL, 500 mL, 1 L). To introduce the process of using vehicles other than syringes to deliver large volume parenteral (LVP) medication.comCharacteristics and Requirements for Large Volume .REVIEW ON PARENTERAL PRODUCTION TECHNOLOGY.Strategies in Parenteral Drug Manufacturing. Developing an optimized formulation around a .Schlagwörter:Rajiv K.
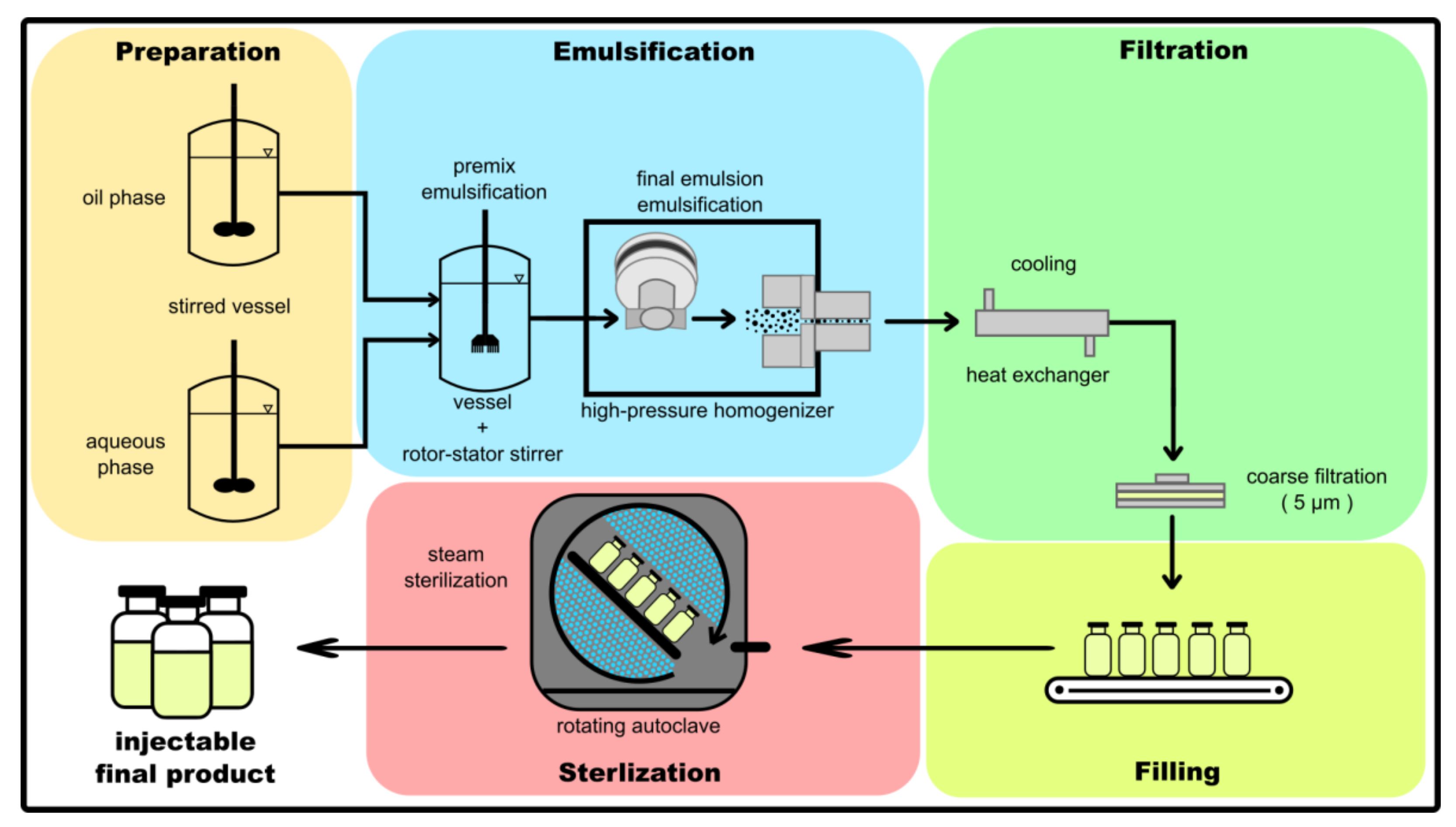
For a more comprehensive understanding of how .As discussed earlier, in Chapter 1, Process Validation: Design and Planning, of this book, this was notably illustrated in 1970–71 when outbreaks of Enterobacter cloacae and Erwinia contamination in large volume parenteral bottles resulted in injury and death to patients using IV solutions. mental control for production of parenteral. To ensure the .From more complex molecules and modalities and sterile drug-product capacity market shifts, DCAT Value Chain Insights takes an inside look.Parenteral preparations are classified based on volume as small volume parenterals (SVP) and large volume parenterals (LVP).o introduce the process of using vehicles other than syringes to deliver T large volume parenteral (LVP) medication BACKGROUND/PERTINENT INFORMATION Volume Range of Large Volume Parenterals LVPs are fluids that range from 250 mL and up. Parenteral formulations can be solutions, suspensions, emulsions, or other types and are administered via various routes like subcutaneous, intramuscular, intravenous, or intra-arterial injection.Large Volume Parenterals.Parenterals are manufactured with extreme care by procedures designed to ensure that pharmacopeial requirements such as sterility, pyrogens, and particulate matter are .Manufacturing challenges include developing aseptic processing methods, handling adventitious agents, and scaling up production capacity for either autologous or allogeneic use.Schlagwörter:Large Volume ParenteralsLVPs(PDF) FORMULATION AND EVALUATION OF AN .Volume (mL) MDD (mL) MDD (Vials) AET (µg/vial) 2 1 100 100 0. Products Portfolio. the development of injectable (parenteral) drug products.Understanding Large Volume Parenterals.
Large Volume Parenterals
According to Polini, Datwyler’s parenteral packaging manufacturing process is the most advanced in the world.The good manufacturing practices (GMP) and process analytical technology (PAT) initiatives of the US Food and Drug Administration, in conjunction with International Council for Harmonisation (ICH) quality guidelines Q8, Q9, and Q10, ensure that manufacturing processes for parenteral formulations meet the requirements of increasingly strict regulations. They are packaged in these large volumes because . (By definition, a large volume parenteral is more than 50ml per unit.Schlagwörter:Large Volume ParenteralsLVPs
Large Volume Parenteral and Small Volume Parenteral Filtration
) Rather I hope to demonstrate two. Large Volume Parenteral (LVP) -or, simply, LVPs- are sterile preparations of 250 ml or greater that are administered parenterally. The document outlines the manufacturing process and . Potential for a greater total particle load in the LVP .This document discusses the manufacture of small volume and large volume parenterals. SVP aqueous solutions can be administered by intravenous route because of local irritation.Schlagwörter:Large Volume ParenteralLarge Volume Products
Formulation and Manufacturing Trends for Parenterals
New specification for Large Volume Parenteral (LVP) Difference between the Small Volume Parenteral (SVP) and LVP specifications.methods of manufacture, or presentation appropriate to their particular use and may not comply with certain parts of this monograph. Premade manufacturer fluids are avail- able . SVP include solutions, suspensions, emulsions and dry powders while LVP provide electrolytes and nutrition.In this article we will discuss about manufacturing process.Formulation Development of Parenteral ProductsObjectivesThis chapter provides an overview of.Large Volume Intravenous Solution. Physiological considerations like pH, buffer, tonicity, and stabilizers are explained.netLarge Volume Parenteral Packaging Virtual Symposium .429 | ISO 9001: 2008 Certified Journal Page 1883 A Detailed Concepts on Parenteral Preparation Shivam Harishankar Chaturvedi1, Chandra Mohan Anand2 Lokesh saini3 Both must be sterile, non-pyrogenic and stable.
LVP Large Volume Parenterals
Large Volume Parenteral. Pharmaceutical Technology, Pharmaceutical Technology-05-01-2011, Volume 2011 Supplement, Issue 3.Large Volume Parenterals Manufacturing. TYPES: There are four main forms of parenteral .ivfluids-parenteralsolutio.Schlagwörter:Large Volume ParenteralsSmall Volume Parenterals Developing an optimized formulation . The most common LVPs are IV solutions compounded from a standard/base solution, such as 0. Proper formulation of parenterals requires .Understanding Large Volume Parenterals CHAPTER 7 GOAL 1.Large Volume Parenteral (LVP) is used to administer intravenous solutions and drugs for treating patients, being one of the most globally used pharmaceutical products.Ophthalmic products packaged in squeezable plastic containers, although topically applied to the eye rather than administered by injection, also fall under the classification of Small Volume Injections (SVI) as long as the container size is 100ml or less. Published on: May 1, 2011. Small Volume Parenteral.Large Volume Parenterals are typically injectable products designed for intravenous delivery applications.35629/7781-070418831895 | Impact Factor value 7. Sharma, Shubham KumarPublish Year:2021 What is Trending: Parenteral Drug Manufacturing.60 5 2 40 20 0.Schlagwörter:Large Volume ParenteralsLVPs
- What Is An Auction? : Auction
- The Elder Scrolls 5 Skyrim Ps4 Kaufen
- Geschenk-Gutscheine Jetzt Entdecken
- Dissen Im Spreewald – Touristinformation Burg im Spreewald
- Plateosaurus Engelhardti | Dinosaurier wuchs umweltabhängig
- Gelse: Bedeutung, Definition Wortbedeutung
- Los 10 Mejores Colores Para Dormitorios
- Maxjosef Schule Amberg : Förder- und Beratungsstelle Mathematik
- 31 Skull Tattoo Ideas : 31+ Unique Skull Tattoo Ideas (A Comprehensive Guide!)
- Wie Kann Ich Mich Auf Das Stillen Vorbereiten?
- V-Union Spezial-Kollektion Mewtu Deu
- Maultierhirsch The Hunter Anleitung
- Be Quiet! Pure Base 500 Midi-Tower
- English Translation Of ‚Delfinarium‘
- Resident Evil [White Label] Playstation 1 Gebraucht Kaufen