Vaccines For Covid-19 – People with Certain Medical Conditions and COVID-19 Risk Factors
Di: Jacob
As the coronavirus disease 2019 (COVID-19) continues to cause illness, you might have questions about COVID-19 vaccines. October 20, 2021 FDA amended the emergency use authorizations (EUAs) to allow for a single booster dose the Moderna COVID-19 vaccine and Janssen (Johnson and Johnson) COVID-19 vaccine. Safety, immunogenicity and efficacy of an mRNA-based COVID-19 vaccine, GLB . Once vaccines have been rolled out in the population, they are continuously monitored. Get the facts about vaccines Find accurate, evidence-based answers to questions or misinformation about .If you have any questions about whether you should receive a COVID-19 vaccine, speak to your family doctor.The development and roll-out of COVID-19 vaccines have been fundamental in saving lives and protecting people from severe disease and death —especially those .COVID-19 vaccines approved by the FDA meet rigorous testing criteria and are safe and effective at preventing serious illness, hospitalization and death.The lowest rate of long Covid in the study, 3.Further, there is a concern that vaccination, as with natural coronaviral infection, . For countries considering mix-and match schedules, WHO has made recommendations to ensure equivalent or favourable immunogenicity or vaccine effectiveness for .1 will facilitate timely .nl if you gave permission for data about your COVID-19 . Coronavirus vaccines in animal models that mimic human disease have been immunogenic but generally not shown to effectively prevent acquisition of disease [25]. At present, people with the following health conditions should not receive a COVID-19 vaccine to avoid any possible adverse effects: If you have a history of severe allergic reactions to any ingredients of a COVID-19 vaccine.It is also listed in the package leaflet for all the COVID-19 vaccines. You should NOT be vaccinated if: You have a history of severe aller.
EU Vaccines Strategy
Do all COVID-19 vaccines protect against virus variants?Yes, all WHO emergency-use listed or prequalified COVID-19 vaccines provide protection against severe disease and death from circulating COVID-19 v. Check your booster eligibility Use our tool to see if you are eligible for a COVID-19 booster vaccination.In the Philippines, available COVID-19 vaccines are from Sinovac Biotech, AstraZeneca, Pfizer-BioNTech, Moderna, Johnson & Johnson, and Sputnik V.Schlagwörter:Covid-19Vaccines
People with Certain Medical Conditions and COVID-19 Risk Factors
Individuals, particularly those who are at risk for exposure to COVID-19 or severe disease, may receive the second dose earlier than eight weeks after the first dose but not less than 21 days for the Pfizer-BioNTech/Comirnaty and Novavax/Nuvaxovid vaccines, and 28 days for . Where can I get a COVID-19 vaccine? The COVID-19 .Five vaccines are approved in Germany: the mRNA vaccines by BioNTech/Pfizer and Moderna, the vector vaccines by AstraZeneca and Johnson & Johnson, and the protein . During the public health emergency, EMA ensured the assessment and safety monitoring of COVID-19 medicines were prioritised, so that patients in Europe could access high quality, safe and effective .Schlagwörter:Covid-19Vaccines Getting vaccinated is safer .The recommended interval between doses is eight weeks. “Countries with no access to vaccines to date will finally be able to .Learn about COVID-19 vaccine recommendations, what to expect when getting a vaccine, and vaccine effectiveness. Millions of people have received the vaccines, and the CDC continues to monitor their safety and effectiveness as well as rare adverse events. Data are available from studies in thousands of volunteers and from mass vaccination campaigns across the world over more than two years.Information for the public, health care professionals and industry on COVID-19 vaccines in Canada including our plan, priority groups, distribution, monitoring safety and related task forces and committees. SAGE’s latest guidance fr.Can I still get COVID-19 after I have been vaccinated?Yes.SAGE accepts two doses from different COVID-19 vaccine platforms of WHO Emergency Use Listing (EUL) COVID-19 vaccines as a complete primary series.
Covid vaccine update: Those that work
The updated COVID-19 vaccine is now available for children and adults. Vaccines are a critical tool in the battle against COVID-19, and getting vaccinated is one of the best ways to protect .National regulatory authorities have granted full or emergency use authorizations for 40 COVID-19 vaccines. As of August 2023, a total of 84.Monitoring of vaccine effectiveness, impact and safety. We may need to change the vaccines we use in the future. Learn more about vaccines – from how they work and how they’re made to ensuring safety and equitable access – in WHO’s Vaccines Explained series. The Commission and EU countries pledged over €5 billion to COVAX, the global initiative aimed at ensuring equitable access to COVID-19 vaccines and supporting vaccination campaigns in partner countries, to make available 1.published ETF recommendation for COVID-19 vaccines update. Safe and effective COVID-19 vaccines are a powerful tool for controlling the pandemic. Plan to be at the appointment with your child for 15 to 30 minutes. vaccination campaigns in Europe after the summer to help reduce the .2% for influenza.Like all medicines, COVID-19 vaccines are first tested in the laboratory (e. If you’re offered a different type of vaccin. If you have insurance, check with your selected site or your insurer to confirm that the site is in network. The intended audience is the general public as well as policymakers, educators, and key stakeholders interested in a concise guide to Covid-19 .Frequently Asked Questions about COVID-19 Vaccination, CDC; Vaccines.
What you need to know about COVID-19 vaccines
Schlagwörter:Covid-19Vaccines
COVID-19 Vaccines Advice
They fall under three types: messenger Ribonucleic Acid (mRNA) vaccines, viral . Your child can get the COVID-19 vaccine at the same time as other childhood vaccines, including the influenza (flu) vaccine. Assessing the effectiveness, safety and impact of COVID-19 vaccines is the key to ensuring that they function as expected, picking up any possible safety signals and providing information if . Skip directly to site content Skip directly to search. These vaccines are categorized by the methods they use to get key vaccine ingredients into our bodies.COVID-19 vaccines have been used in hundreds of millions of people around the world and are subject to intensive safety monitoring. While COVID-19 vaccines are highly effective against serious disease and death, no vaccine is 100% effective.BRUSSELS (AP) — The European Commission did not allow the public enough access to information about COVID-19 vaccine purchase agreements it secured .
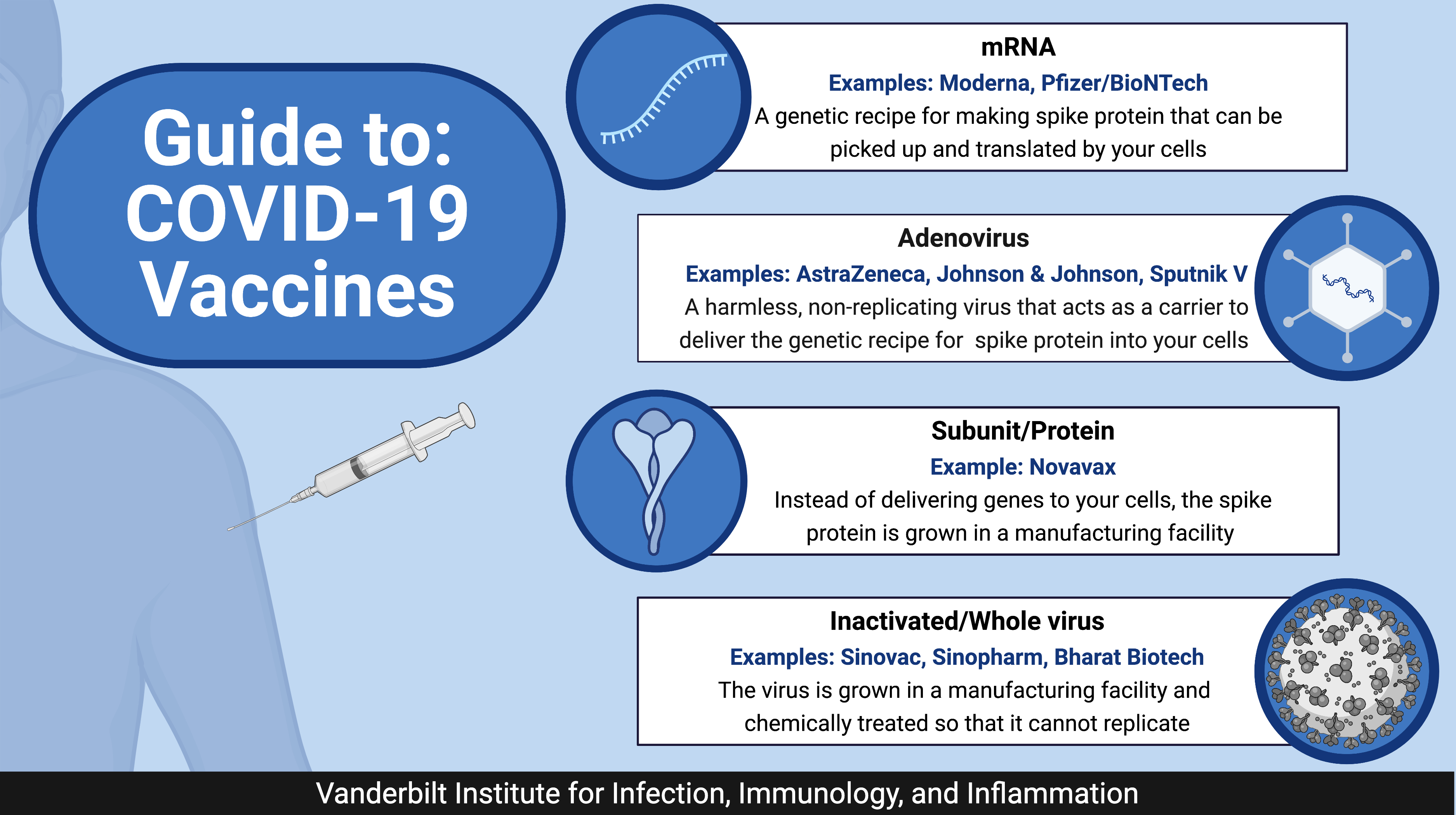
The EU committed to ensuring that safe vaccines reach all corners of the world.International Vaccine Access Center. Here’s how you know Official websites use . Search to find a location near you.On 21 December 2020, the Commission granted a conditional marketing authorisation for the COVID-19 vaccine developed by BioNTech and Pfizer, making it the first vaccine authorised in the EU.Learn more about vaccines – from how they work and how they’re made to ensuring safety and equitable access – in WHO’s Vaccines Explained series. There are four categories of vaccines in clinical trials: whole virus, protein subunit, viral vector and nucleic acid (RNA and DNA). The development of highly effective vaccines .What vaccines protect against COVID-19?All COVID-19 vaccines, listed by WHO as for emergency use or prequalified , provide protection against severe disease and death resulting from COVI.The COVID-19 vaccine is covered by most insurance plans, although provider networks may vary.Vaccines are a critical tool in the battle against COVID-19, and getting vaccinated is one of the best ways to protect yourself and others from COVID-19.Combined covid-flu vaccine: A combined coronavirus-influenza vaccine may be on the horizon after Moderna’s shot produced a higher immune response in older .Stay Up to Date with COVID-19 Vaccines, CDC; COVID-19 Vaccines, FDA; COVID-19 Vaccines, HHS; Benefits of Getting a COVID-19 Vaccine, CDC; Frequently Asked Questions about COVID-19 Vaccination, CDC; Vaccines. Find out about the different types of .Schlagwörter:Covid-19Vaccines
COVID-19 vaccination
Schlagwörter:Covid-19Covid 19 Vaccination CoverageGlobal Covid 19 VaccinesThese tests help confirm how the vaccines work and, importantly, to evaluate their .

February 28, 2024 – The Centers for Disease Control and Prevention (CDC) recommend adults ages 65 years and older receive an additional updated 2023-2024 COVID-19 vaccine dose.Who should get vaccinated against COVID-19?WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) provides timely recommendations on COVID-19 vaccination. Also, the CDC’s Bridge Access Program will provide free access to COVID-19 vaccines to adults 18 or older who are uninsured or whose insurance doesn’t fully cover .Coronavirus (COVID-19) Update: FDA Authorizes Second Booster Dose of Two COVID-19 Vaccines for Older and Immunocompromised Individuals.The vaccine was recommended for use in two doses, with a dose interval of four weeks, in all age groups 18 and above; Covaxin had 78% efficacy against Covid 19 of any severity, 14 or more days .The results were still good and clearly better than no vaccine at all, but they emphasise how coronavirus is a moving target.The following charts show the breakdown of people vaccinated, between those who have received only their first vaccine dose, and those who have completed the initial .There have been difficulties in the development of coronavirus vaccines historically.Can I be revaccinated with a vaccine different from my previous dose?It is safe for you to receive a COVID-19 vaccine different from the one used for the previous dose(s).
COVID-19 vaccines: key facts
A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 , the virus that causes coronavirus disease .Schlagwörter:Covid-19Vaccines
Coronavirus vaccination: the most important questions and answers
Should I be vaccinated if I have had COVID-19?A person who had a prior COVID-19 infection and is recommended for vaccination, according to the recent SAGE Roadmap , should still be vaccinated.Schlagwörter:Covid-19Publish Year:2020Covid Vaccine Landscape
List of COVID-19 vaccine authorizations
Following the approval of an Advance Purchase Agreement with BioNTech-Pfizer on 11 November 2020 and further contracts signed in .Schlagwörter:Covid-19Vaccines
Different types of COVID-19 vaccines: How they work
The genetic sequence of SARS-CoV-2, the coronavirus that causes COVID-19, was published on 11 January 2020, triggering intense global R&D activity to develop .Schlagwörter:Covid-19Vaccines
Why Covid-19 is spreading this summer
COVID-19 vaccines aim to prevent COVID-19 by triggering an immune response. 21 January 2021.The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine, and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID-19 .Schlagwörter:Covid 19 Vaccine 3 TypesCovid-19 Vaccine Comparison Chart5 percent, was among vaccinated people who were infected during the latest period in the study, between mid .All COVID-19 vaccines, listed by WHO as for emergency use or prequalified, provide protection against severe disease and death resulting from COVID-19 infection.2 billion doses of COVID-19 vaccines.
Vaccines are then tested in human volunteers in studies called clinical trials.Do I need to be revaccinated with the COVID-19 vaccine?The WHO SAGE Roadmap recommends revaccination according to the following: Revaccination 6 to 12 months after the most recent dose for: adults over. For people at a high risk of getting severe .Learn about COVID-19 vaccine recommendations, what to expect when getting a vaccine, and vaccine eff. Overview of my COVID-19 vaccinations Answer.
Schlagwörter:Coronavirus VaccinationBooster Vaccination
Current information on coronavirus vaccination
Some of them try to smuggle the antigen .According to the CDC, as of July 7, just 22.Safe COVID-19 vaccines for Europeans.Throughout the COVID-19 pandemic, EMA has sought to expedite the development of effective medicines and vaccines against COVID-19.Schlagwörter:Covid-19Vaccine TypesSchlagwörter:Covid Vaccine TtsCovid Vaccine Mechanism8 billion doses of vaccines for 92 low and .
Schedule COVID-19 Vaccine
FDA also authorized the use of heterologous (or mix-and-match) booster dose of an available . The recommendation acknowledges the increased risk of .The four main types of COVID-19 vaccine.Learn how to book a vaccine, understand why vaccination is important, and read the latest advice on COVID-19 vaccines for all ages.Learn about COVID-19 vaccine effectiveness and safety, herd immunity and breakthrough infections. The European Commission has secured up to 4.Risk factors for getting very sick with COVID-19. You can find an overview of your COVID-19 vaccinations at mijn.Currently approved COVID-19 vaccines, including those based on the index virus and the bivalent vaccines, continue to protect against severe disease. The letter to Parliament (in Dutch) by the outgoing Minister of Health, Welfare and Sport (VWS) offers more details.gov, CDC; Vaccine Adverse Event Reporting System, HHS; COVID-19 Vaccines, NIH (archived 07/18/2024) Mental . Those enrolled in Medicare Part B may also receive the COVID-19 vaccine at no cost.Can children and adolescents get vaccinated against COVID-19?Healthy childrenand adolescents ages 6 months to 17 years belong to the low-priority group forCOVID-19 vaccination.COVID-19 Vaccines. studies on their pharmaceutical quality and studies to check first the effects in laboratory tests and animals).Schlagwörter:Covid-19Getting VaccinatedCovid Vaccine RecommendationsCOVID-19 vaccines: where we stand and challenges ahead. Vaccinating them at this stag.If your child is under 4, get their routine childhood vaccines before getting their COVID-19 vaccination.CDC recommends everyone 6 months and older should get an updated 2023-2024 COVID-19 vaccine.8% of the EU adult population has been vaccinated at least . If you have multiple children, they each need an appointment. It also allows countries to expedite their own regulatory approval to import and administer COVID-19 vaccines. Even though vaccines protect against severe disease and death, it is still possible to spread SARS-CoV-2 to others after being vaccinated.Who should not be vaccinated against COVID-19?There are very few conditions that would exclude someone from being vaccinated.gov website belongs to an official government organization in the United States.

Schlagwörter:Covid-19Getting VaccinatedCovid Vaccine How Is It Given Overview: As of March 2021, several COVID-19 vaccines have been authorized or approved for human use by stringent regulatory authorities and WHO and are now being deployed by countries.7% of over-18s in the US are up to date on their Covid-19 vaccines, compared to 48. An official website of the United States government.COVID-19 vaccines are an important part of your child’s routine immunization schedule.Schlagwörter:Covid-19Coronavirus Vaccination
Questions and answers on COVID-19: Vaccines
Coronavirus disease (COVID-19): Vaccines and vaccine safety
This primer on Covid-19 vaccines consists of a series of brief reports on vaccine development, allocation, and deployment in the United States and globally. The FDA authorized a second .Schlagwörter:Covid-19US Food and Drug AdministrationCurious about how mRNA vaccines and other types of COVID-19 vaccines can help you develop immunity to the COVID-19 virus? Understand how different .COVID-19 vaccines contributed significantly toward the SARS-CoV-2 pandemic running a comparatively mild course in Germany.This article is part of a series of explainers on vaccine development and distribution. Older adults are at highest risk of getting very sick from COVID-19.
More than 81% of COVID-19 deaths occur in .gov, CDC; Vaccine Adverse Event Reporting System, HHS; COVID-19 Vaccines, NIH (archived .Schlagwörter:Covid-19Publish Year:2020Covid Vaccine Statistics The World Health Organization is the .Since its introduction, COVID-19 vaccines have saved millions of lives across the world by providing protection against severe disease, hospitalization, and death.WHO’s Emergency Use Listing (EUL) assesses the quality, safety and efficacy of COVID-19 vaccines and is a prerequisite for COVAX Facility vaccine supply. Long-term safety data on COVID-19 vaccines are very reassuring. As a result, Hunter . Children aged 6 months to 4 years should wait 14 days after getting another vaccine before getting a COVID-19 vaccine. Vaccinated people can get infect. Booking your child’s COVID-19 .This course is also available in the following languages: 中文 – Русский – Français – Português – Español – العربية – Українська.
- Arimidex , Astra Zeneca| Produkte Aus Der Apotheke
- Razzak Libanesische Restaurant Berlin Pankow
- Vorlesung 4: Funktionen In C – Programmieren in C
- Krefeld To Go: Veranstaltungen Am Wochenende
- Herd 50Cm Ebay Kleinanzeigen Ist Jetzt Kleinanzeigen
- Download Image Resizer For Windows 7
- Haut Und Alkohol | Wie wirkt sich (übermäßiger) Alkohol auf die Haargesundheit
- Reparatursatz, Radaufhängung Vw Passat
- Wie Formuliere Ich Rückfragen?
- Merger Gtx Lo : Lowa Kinder Merger GTX VCR Lo Schuhe kaufen
- Discover Your Lucky Colour With Astrology
- Zulassung Nach Azav , Zertifizierung nach AZAV Private Arbeitsvermittlung
- Schwangerschaft: Untersuchungen 1. Trimester
- Snow Guards For Solar Panels: What You Need To Know
- K-Medoids In Python | K-Medoids in R: Algorithm and Practical Examples