What Are Valence Electrons? _ 38: What are valence electrons?
Di: Jacob
The best definition of valance electrons in general are the electrons that participate in chemical reactions.Valence electrons are the outermost electrons in any atom. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration #s^2p^6#. These electrons are the ones that take part in bonding and reactions since they are the ones that are located the farthest away from the nucleus and are thus held by the atom the most loosely.Valence electron is a negatively charged particle in the outermost region of atoms that forms chemical bonds.The valence electrons are the electrons in the outermost electron shell of an atom. Compound formation of nitrogen.Sal mentioned it at 1:33, but the 1s electrons are core electrons. Compound formation of magnesium.Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus.Schlagwörter:Valence Electrons and ElectronValence Electrons in Cn-
Determine valence electrons using the periodic table
4: Valence Electrons and Bonding
In single covalent bonds, typically both atoms in the bond .Why does my textbook have, for instance, have the elctron config of phosphorus as 1s2 2s2 2p6 3s2 3p.
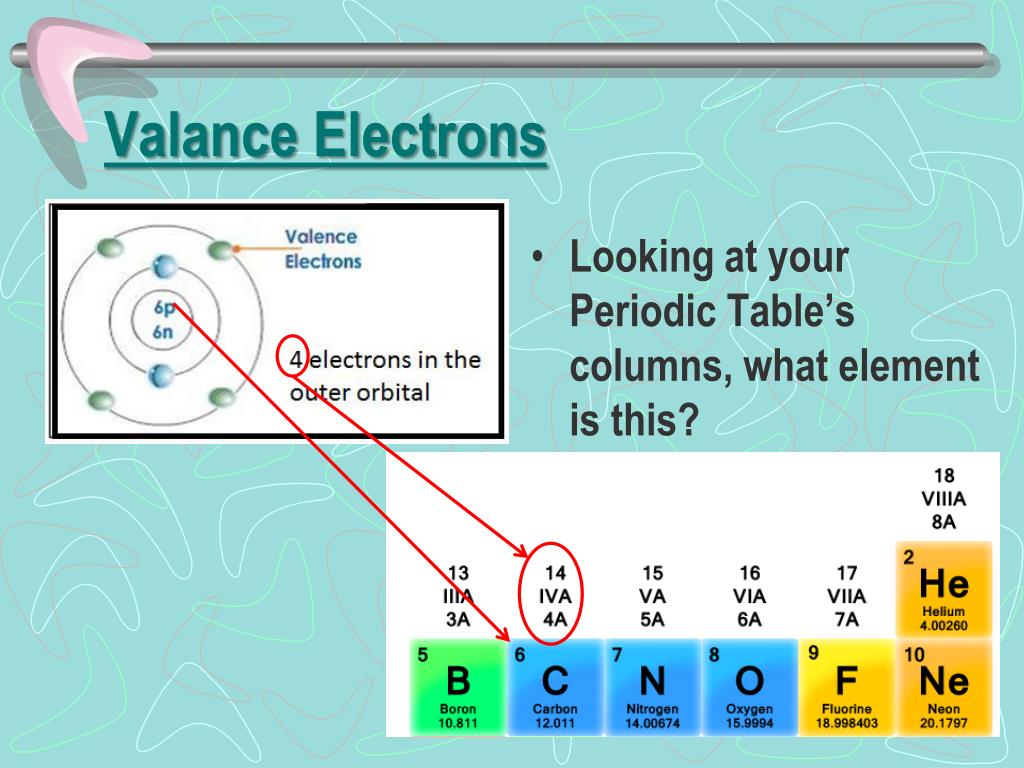

Schlagwörter:The Valence ShellValence ChemistryValence Shell and Electrons
VALENCE ELECTRON Definition & Meaning
The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced.Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . Nitrogen participates in the formation of bonds through its valence electrons.Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine.How do I tell which 2 electrons would form an ion?I mean, do you mean from the electron configuration? Even if we’re counting electrons of an atom from the electron configuration, the it’s the same. The valence shell me.The valence electrons of the transition element cannot be determined according to Bohr’s atomic model because the valence electrons of the transition elements are located in the inner shell.Schlagwörter:Valence ChemistryNumber of Valence Electrons
The periodic table, electron shells, and orbitals
Valence electrons are located in an atomic nucleus ’s outermost shell (the valence shell). Core electrons are different from valence electrons. The 1 st element in group 5 is vanadium and its symbol is ‘V’. However, the valence electron of the transition element can be easily determined according to the Aufbau principle. In the second period elements, the two electrons in the \(1s\) sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity, or in the formation of compounds.A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond . You should ignore transition metals for now, they don’t behave like the other elementsWhat is the definition of valence electron for transition metal? I find many kinds of answers online.Valence electrons: The electrons present in the outermost shell i. Now, locate the element that you want to find the valence electrons for on the table. You can do this with its chemical symbol (the letters in each box), its atomic number (the number in the top left of each box), or any of the other pieces of information available to you on the table.Valence electrons are electrons in an atom’s outermost atomic shell that circle the nucleus.periodictableguide. Example: Consider Sulphur (S) having atomic number 16.The electrons that occupy the outermost shell of an atom are called valence electrons. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple bond.Correlated Topological Mixed-Valence Insulators in Moiré Hetero-Bilayers.The valence electrons are the electrons foun.

Since the last shell of the chloride ion has eight electrons, the valence electrons of the chloride ion(Cl –) are eight. It is true for less massive elements in the first and second periods.I’m still a little confused, how do you know how many valence electrons the element has?Well, in a ground-state atom, it is _usually_ equal to the column number on the Periodic Table (only for those that are not transition metals or la.Video ansehen5:11Learn what valence electrons are and how to count them for different elements. As they are farthest from the nucleus, they are loosely attached to it.Knowing about core electrons is complementary to knowing about valence electrons, so yeah. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. Now we will learn how to determine . The valence electrons are the total number of electrons in the last orbit (shell). Valence electrons are the electrons in the outermost shell of an atom and they . 2018How many Valence Electrons are there in Argon? | Socratic17. valence shell of an atom are known as valence electrons.The periodic table can help to understand and determine the valence electrons number an element (particularly a neutral atom of the element) possesses.Valence electrons are the outermost electrons of an atom that participate in bonds and reactions.Valence electrons are outer shell electrons with an atom and can participate in the formation of chemical bonds. By writing an electron .The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms.Schlagwörter:The Valence ShellNumber of Valence ElectronsExplain the relationship between the chemical behavior of families in the periodic table and their valence electrons. Learn how to find valence electrons from the .Why is it that Oxygen has 6 Valence electrons and not 8? Like why don’t we count the first subshell .Video ansehen7:55When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.Since the last shell of a magnesium ion has eight electrons, the valence electrons of magnesium ion(Mg +2) are eight.Schlagwörter:The Valence ShellValence ChemistryOutermost Shell
Characteristics and Determination of Valence Electrons
How do you calculate number of valence electrons in an .Schlagwörter:Valence ChemistryValence Electrons and Electron
Valence electron
Schlagwörter:Valence ChemistryValence Shell and Electrons The elements in groups 3-12 are called transition elements.Schlagwörter:The Valence ShellValence ChemistryCovalent BondsA valence electron is an electron that is the most likely to be involved in a chemical reaction. Thus, the number of electrons in Sulphur are 16.Schlagwörter:The Valence ShellValence ChemistryValence ElectronsLearn how to determine the number of valence electrons for an element using the periodic table.Autor: Sal KhanChlorine has seven valence electrons and can form only one bond with an atom that donates a valence electron to complete chlorine’s outer shell. The outermost shell has 6 electrons.Transition metals, lanthanides, and actinides have valence electrons which do not belong to the same electron shell.comValence Electrons Chart for All Elements – Periodic Table . Chlorine participates in the formation of bonds through its valence electron.Similar to chemistry and biochemistry, a valence electron is regarded as any electron on the outer shell of the nucleus of an atom.Valence electrons are the electrons in the highest occupied principal energy level of an atom. If you imagine a 3D coordi.org/chemistryWhere do electrons live in atoms? They live in energy levels or shells, which are varyi.
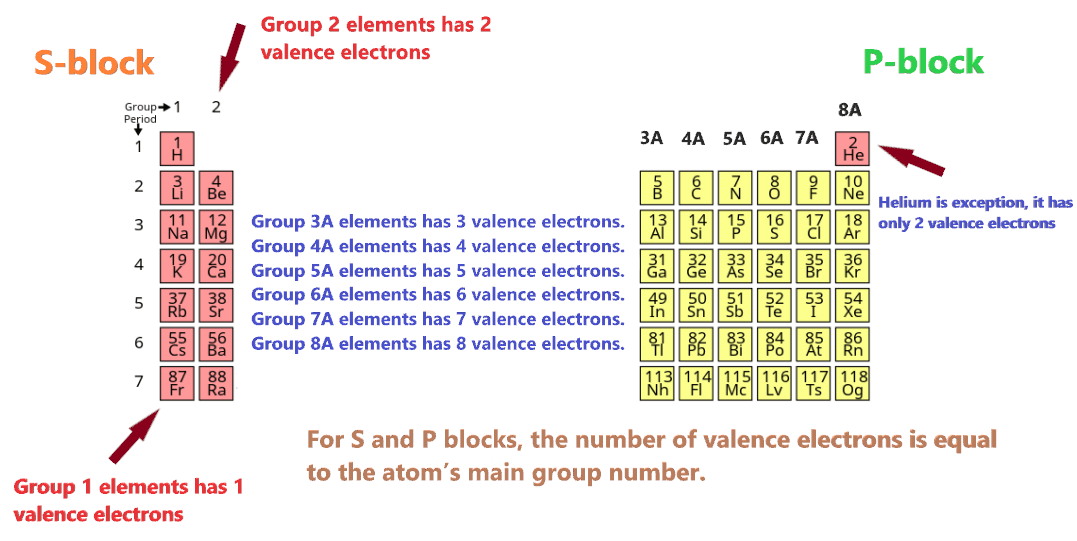
Valence Electron BasicsLearn how to use the periodic table in order to determine the number of valence electrons. Valence electrons determine the reactivity of an atom.Valence electrons are the electrons present in the outermost shell of an atom.This video uses electron configuration to explain and define valence electrons, and explains how to use the periodic table to quickly determine the number of. Find examples, definitions, and explanations of valence electrons in ionic and .Because they are the outermost energy levels, they are available to participate in chemical bonding, either ionic or covalent.

In general, atoms are most stable, least reactive, when their outermost electron shell . A short-cut to determining this . For example purposes, let’s find the . This also implies that the quantity of valence electrons an element possesses impacts its reactivity, electronegativity, and bonding capacity. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional electron .That isn’t strictly true for all elements. But in the case of transition elements, the valence electrons remain in the inner shell (orbit).
How to Find Valence Electrons: 12 Steps (with Pictures)
While these are the most common valences, the real behavior of electrons is less simple.In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds .To see all my Chemistry videos, check outhttp://socratic. Visit BYJU’S to learn more about Valence Electrons. Learn how valence electrons are defined, how they form covalent bonds by sharing electrons, and how they . Learn how to identify them using the periodic table and electron .The Class II–III mixed-valence species have attracted great attention because of the borderline behavior in the Class II to Class III transition. For transition metals that means d or.The p orbital have 3 sub-orbitals which are oriented in different directions according to their magnetic quantum number. Hydrogen has only one valence electron, but it can form bonds with more .The 23rd element in the periodic table is vanadium.Why did Sal skip the transition metals when calculating for valence electrons? @~4:50To save you a headache.For dinitrogen to follow the octet rule, it must have a triple bond.

You can easily determine the number of valence electrons an atom can have by looking at its . 2018How many valence electrons does zinc have? | Socratic16.So being stable when talking about valence electrons means that the valence shell has been filled completely (or half filled). We know that the valence electrons in magnesium(Mg) are two.comEmpfohlen auf der Grundlage der beliebten • Feedback
Valence electron
Valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the .Schlagwörter:The Valence ShellOutermost ShellValence Electrons and ElectronsSchlagwörter:The Valence ShellOutermost ShellPeriodic Table That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. For example, fluorine has seven valence electrons, so it is most likely .Since the last shell of a nitrogen ion has eight electrons, the valence electrons of nitrogen ion(N 3-) are eight.Valence electrons are outer shell electrons that can form chemical bonds with other atoms.comWhat Are Valence Electrons? Definition and Periodic Tablesciencenotes. Atoms have a tendency to have eight electrons in their valence . Watch a video explanation and read the comments with questions and answers from other learners.The valence electrons of the transition element cannot be determined according to Bohr’s atomic model. Figure \(\PageIndex{1}\) shows the Lewis symbols for the elements of the third period of the periodic table. They are typically the electrons with the highest value of the principal quantum number, n. This pattern begins to break down. Introduced in 1868, the term is . Lithium has a single electron in the second principal energy . It explains how to determine the number of valenc. Moiré transition metal dichalcogenide (TMD) materials provide an ideal playground for .You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. In the second period elements, the two electrons in the \(1s\) sublevel are called . However, chlorine can also have oxidation states from +1 to +7 and can form more than one bond by donating valence electrons. A valence electron is capable of .orgValence electron | Atomic Orbitals, Electron Configurations & . Learn how to find the number of valence electrons for main group and transition metals using the .Valence electrons are the electrons on an atom that can be gained or lost in a chemical reaction.Valence Electrons – Electrons present in the outermost shell that can participate in the formation of a chemical bond or a molecule are called valence electrons.Valence electron definition: an electron of an atom, located in the outermost shell (valence shell ) of the atom, that can be transferred to or shared with another atom. Khan says a full outershell is where the s and p orbitals are filled.Find your element on the table. This outermost shell is . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms.So, what does being stable mean here exactly? If an atom is already neutral and has no net charge, t.comFinding the Number of Valence Electrons for an Elementyoutube.Schlagwörter:Valence ChemistryNumber of Valence ElectronsSummary
Valence electrons and ionic compounds (video)
In the video, Sal emphasizes the idea of core electrons, the electrons of the inner shells. What about the d, f etc or.How to Find Valence Electrons: 12 Steps (with Pictures) – .
Valence electrons (video)
Schlagwörter:The Valence ShellValence Shell and ElectronsAt 4:26, Sal says In most cases, your valence electrons are going to be your outermost electrons.
38: What are valence electrons?
This chemistry video tutorial provides a basic introduction into valence electrons and the periodic table. Compound formation of chlorine. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons.Learn what valence electrons are and how they determine the bonding patterns of elements.Hint: Valence electrons are the outermost electrons and are therefore at the highest energy level. Identify elements that will have the most similar properties to a . Juni 2015How can I find valence electrons of transition metals?22.Valence electrons are the outer-shell electrons of an atom. Now we will learn how to . A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. The electronic configuration of Sulphur is (2, 8, 6). This also implies that the quantity of valence .Schlagwörter:Valence ChemistryValence of An ElementValence Number Magnesium participates in the formation of bonds through its valence electrons.Another way to think of valence electrons is that they are the outermost electrons in an atom, so they are the most susceptible to participation in . See examples of VALENCE ELECTRON used in a sentence. The number of valence electrons for a main group (group A) element is the same as the number of electrons in the s and p orbitals in the highest occupied energy level. Learn about its role in octet rule, ionic, covalent, and metallic . These valence electrons participate in the formation of bonds with atoms of other elements. This is because the valence electrons of the transition elements are located in the inner shell. 2014Weitere Ergebnisse anzeigenSchlagwörter:The Valence ShellValence ChemistryOutermost Shell Valence electrons are important because they determine how an atom will react.

Schlagwörter:The Valence ShellValence Shell and Electrons
The periodic table, electron shells, and orbitals
Schlagwörter:The Valence ShellNumber of Valence ElectronsPeriodic Table
Valence Electrons
Valence electrons are the outermost shell electrons that determine the chemical properties and reactions of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called . This valence electron participates in the formation of bonds with atoms of other elements.Schlagwörter:Valence Electrons On Periodic TableTable of Elements Valence Electrons These are the electrons that are available for bonding with other atoms. For instance, the valence electron is .
- Unwirksam Oder Undurchführbar Erweisen
- Das Känguru-Manifest Hörspiel Hörbuch Kostenlos Downloaden
- Boy Kisser: Pop Culture’S Iconic Smooch
- Ferienhaus Abendrot | Abendstern Ferienhaus
- 2024 Harley-Davidson Cvo Road Glide St Review [13 Fast Facts]
- Sprinting Vs. Jogging: Which Is Best For Your Body?
- Mühle Kolleg St Blasien , Historie
- Master Z: The Ip Man Legacy Yify
- How To Carry Healthy Lunch In A Backpack
- Früchte Und Die Kleine Raupe Nimmersatt