What Is Calcium Carbonate _ Calciumcarbonat
Di: Jacob
Calcium Carbonate.Calcium carbonate is used to prevent or to treat a calcium deficiency.Medizinisches Calciumcarbonat, bekannt als Calcium Carbonate, ist ein Arzneimittel, das im Rahmen der Calciumergänzung bei Erkrankungen des .

In some cases, the presence of sodium is objectionable so that the ammonium carbonate salt is preferable. Calcium Carbonate is Mildly Basic At concentration of 1 mM, it has a pH of 9.Calcium carbonate minerals are involved in the global carbon cycle and have been intensively investigated . Paper Calcium carbonate is used in the production of paper as a filler and a coating material to improve the paper’s brightness, smoothness, and other . Contra-indications. Indications and dose.
Calcium Carbonate: MedlinePlus Drug Information
Calcium carbonate also occurs combined with magnesium as the mineral dolomite, CaMg (CO 3) .91 At concentration of 100 mM, it has a pH of 9.Calcium carbonate is an inorganic salt primarily used to manage and treat low calcium conditions, GERD, CKD, and other indicated conditions.Calcium Carbonate is a chemical compound having the chemical formula CaCO3.Calcium carbonate. Calcium carbonate is characterized by its unique chemical structure, represented by the formula CaCO₃.Calcium Carbonate Facts | BICCF. Contains the highest concentration of elemental calcium at 40% by weight. It is the primary component in the shells of . Omya’s natural mineral products, complemented by our broad portfolio of specialty materials and expert technical advice, help customers to manage process efficiencies and deliver high-performing products with a lower carbon .In this Forfar Minisode, Educational Staff Haleigh Collins gives a quick overview of Calcium Carbonate and it’s importance. It may contain considerable amounts of magnesium carbonate (dolomite) as well; minor constituents also commonly present include clay, iron carbonate, feldspar, pyrite, and quartz. Calcium Acetate is a white crystalline powder that is highly soluble in water, making it suitable for pharmaceutical and medical uses, such as in the treatment of hyperphosphatemia. Calcium carbonate occurs in two main forms—the hexagonal crystal known as calcite, and the orthorhombic form, aragonite.Geschätzte Lesezeit: 1 Minuten
Calcium Carbonate
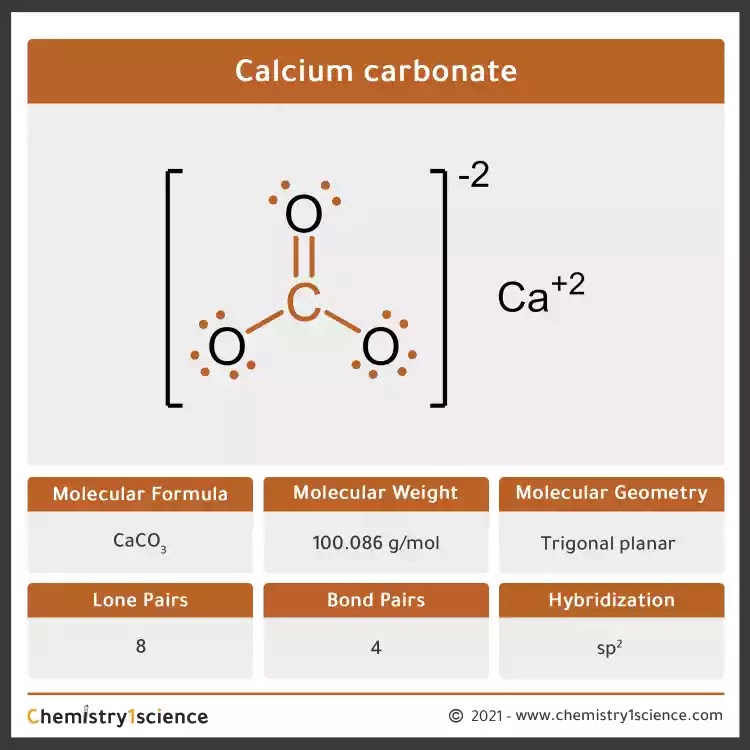
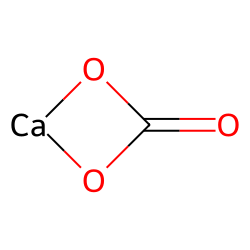
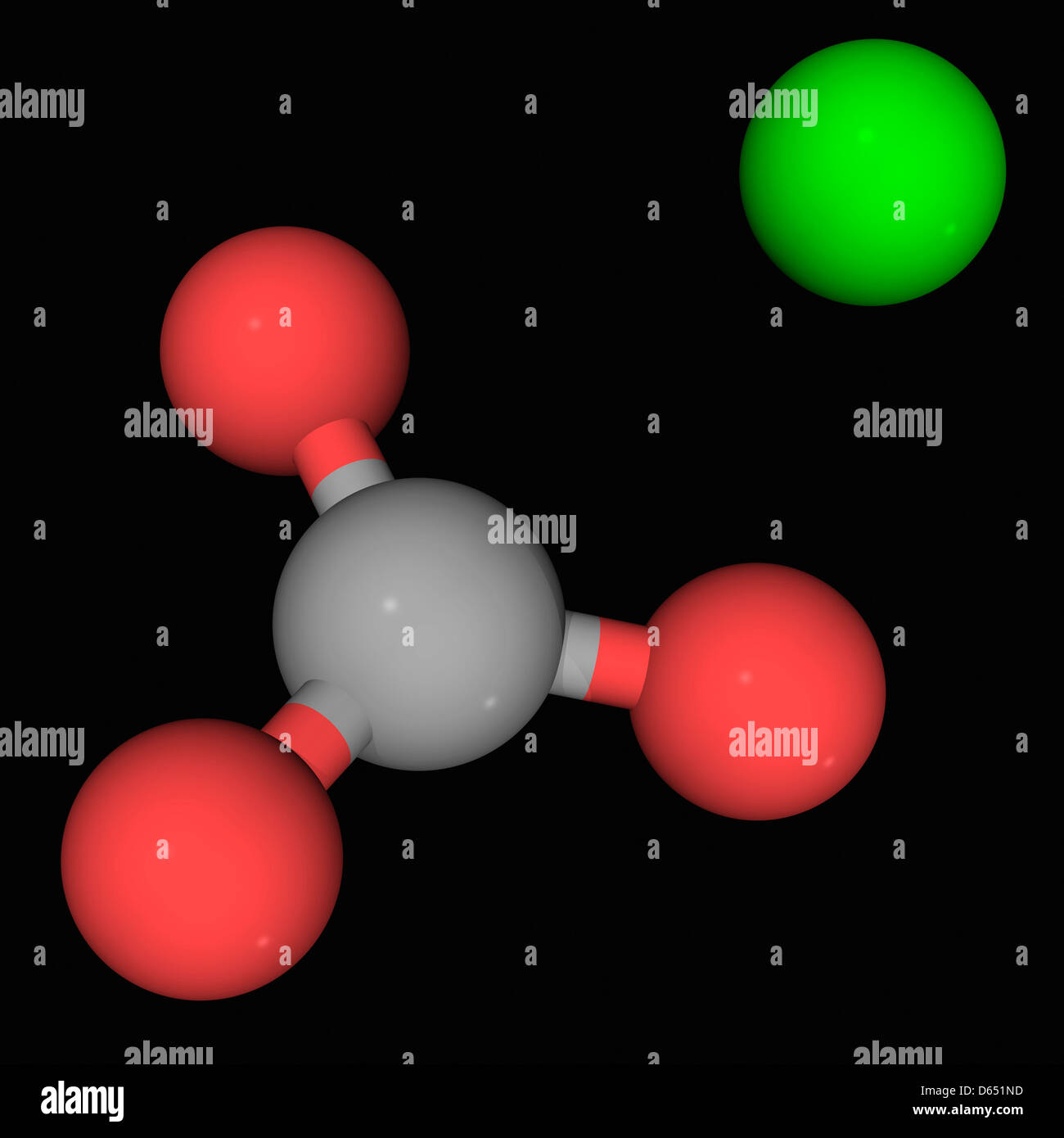
Alkali metals can be mined in the form: Na 2 CO 3, sodium carbonate.At its core, this compound consists of one calcium (Ca) ion bonded to one carbonate (CO₃) ion. The MFR method produces ACC with consistent compositions that systematically correlate with the solution chemistry conditions without evidence of .Calcium Carbonate, denoted chemically as CaCO 3, is a common substance typically found in rocks across all parts of the globe.

Tablets are often smaller and fewer are needed to meet the .Calcium carbonates are one of the most abundant minerals in the earth’s crust.In der Systematik der Minerale nach Strunz (9. Clicking each .
What is calcium carbonate and what is it used for?
Calcium Carbonate
As for its safety, it’s probably one of the safest ingredient in an oral care product. Symptoms can be alleviated by switching to a supplement containing a different form of calcium, taking smaller calcium doses more often during the day, or taking the supplement with meals.Find patient medical information for calcium carbonate on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings. It occurs in a wide variety of mineral forms, including limestone, marble, travertine, and chalk.Calcium carbonate is typically used in the form of limestone to correct soil Ph for lawns. Except for Li 2 CO 3, alkali metal carbonates are thermally stable.Calcium carbonate appears to cause more of these side effects than calcium citrate, especially in older adults who have lower levels of stomach acid . Sodium Carbonate (soda ash) is used in the production of glass. The number of various possible uses of CaCO 3 is really impressive! At present, calcium carbonate is also used: to relieve heartburn or acid stomach, as an intermediate in the production of chalk, as an ingredient of bleaches, as a pigment.
Calcium
Follow the directions on your prescription or package label carefully, and ask your doctor or pharmacist to explain any part you do not understand. It is a water-insoluble source of calcium.Carbonate is a moderately strong base. Calcium carbonate is classified as a calcium . Right: cross-section through a sea urchin spine, which is also a single crystal of calcite. Over 20% of the World’s sedimentary rocks are composed of chalk or limestone.Overview
Calcium carbonate
Calcium carbonate is one of the most useful and versatile materials known to man. Left: single crystal of geological calcite. The carbonate ion is a polyatomic ion with a trigonal planar geometry, comprising one carbon atom centrally . The coating material sticks to the .Calcium carbonate is a chemical compound, with the chemical formula Ca C O 3.Autor: The Editors of Encyclopaedia Britannica
Calcium Carbonate: Uses, Dosage, and Potential Side Effects
Specifically, though Calcium carbonate is not soluble in water, it readily dissolves in dilute Hydrochloric .Calcium carbonate is an odourless chemical compound.It is one of the most common and widely distributed rocks on Earth, with a wide range of uses in various industries and natural settings. It is also used as a calcium supplement and to .Calcium carbonate: This contains 40% elemental calcium. It forms carbonate ions that react with free hydrogen ions to raise the system’s pH. Some over-the-counter antacids, such as Tums and Rolaids, also contain calcium carbonate.Calcium carbonate is a common toothpaste ingredient with 5 key functions: whitening abrasive, white colorant, thickener, remineralization, and oral buffering. The calcium carbonate coating process includes steps that must be done carefully.Carbonate kommen überall in der Natur vor, meist in Form verschiedener Minerale. Calcium carbonate makes up 4% of the earth’s crust. It is mainly found in rocks and is the carbonic salt of calcium.Calcium Carbonate is a salt of calcium that, when dissolved in water, increases the alkalinity of the solution.Limestone, sedimentary rock composed mainly of calcium carbonate, usually in the form of calcite or aragonite.Calciumcarbonat ist ein weißer, farb- und geruchloser Feststoff, der in Säuren unter Entwicklung von Kohlendioxid gut löslich ist. You can see, carbon dioxide gas is released through the solution. It is a white and insoluble powder-like substance that occurs naturally in minerals, marble, chalk, limestone, shells, calcite, pearl, and other related compounds. In organischen .
calcium carbonate (kar-bŏ-nayt) n.Calcium carbonate is absorbed best when taken with food. When used in higher concentrations, it imparts white color to the food and can act as bulking aid.Calcium carbonate is a chemical compound with the formula CaCO₃. It is produced by the sedimentation of the shells of small fossilized snails, shellfish, and coral over millions of years.Calcium carbonate: fun facts. Other forms of .

It is a common substance found in rocks as the minerals calcite and aragonite (most notably as .Calcium carbonate (CaCO3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the .Precipitated calcium carbonate (CAS: 471-34-1) is produced industrially by the decomposition of limestone to calcium oxide followed by subsequent recarbonization or . It reacts with acid to neutralize and release CO2.Besides the so-called ground calcium .
On the other hand, Calcium Carbonate is a white .
Calciumcarbonat
It is polymorphous (same chemical formula but different crystal structure) with the minerals aragonite and vaterite and with several forms that apparently exist only under .Calcium carbonate (CaCO 3) is a substance widely used for various purposes, for example, as a filler and pigment material not only in paper, plastics, rubbers, paints, and inks but also in pharmaceutics, cosmetics, construction materials, and asphalts and as a nutritional supplement in animal foods (1). Afterward, a coating material, such as a surfactant or polymer, is added to the mixture.What is Calcium Carbonate? Calcium carbonate is an inorganic chemical compound with the chemical formula CaCO 3. They occur in the form of limestone and chalk, formed from fossils, and marble, formed from the . Calcium citrate is absorbed well on an empty stomach or a full stomach.
Calcium and calcium supplements: Achieving the right balance
Calcium Acetate is the calcium salt of acetic acid, while Calcium Carbonate is the calcium salt of carbonic acid. a salt of calcium that neutralizes acids and is used in many antacid preparations.
Calciumcarbonat
If you have overly acidic soil, this nutrient will raise soil pH to a more optimum level for growing grass. Medicinally, we use it as an antacid or a calcium supplement. People with low levels of stomach acid—a condition most common in older people—absorb calcium citrate more easily . Chemical formula: CaCO 3.It is known for being virtually insoluble in water but soluble in acidic solutions, making it useful for agricultural soil conditioning and as a safe snow-melting agent due to . It must be taken with food because it needs stomach acids for the body to absorb it. This family of essential minerals comprises more than four percent of the earth’s crust and is found worldwide. Also known as: Limestone, calcite, aragonite, chalk, marble, pearl, oyster shell Saturated.Auflage) bilden sie zusammen mit den . It contains 40% elemental calcium, the highest amount in supplement form for maximum absorption. Not all brands are .
What is Calcium Carbonate?
The many lives of calcium carbonate
This type is commonly available, and it is relatively cheap and convenient. Calcium carbonate is one of the most popular chemicals . Take calcium carbonate exactly as directed. The best way to apply calcium carbonate, aka lime, to your existing lawn, is simply to apply a liquid lime or granules to the top of the grass.Calcium carbonate is often used as a dietary supplement in the form of calcium pills, and it is also added to some food products, such as flour and cornmeal, to increase their calcium content. Tiny calcium carbonate particles are finely ground and mixed with a liquid like water or a solvent. Some of the pure .Limestone is a sedimentary rock primarily composed of calcium carbonate (CaCO3) in the form of mineral calcite or aragonite. Prescribing and dispensing . Limestone forms through the accumulation and compaction of marine .Calcium carbonate coating process. It is usually taken three or four times a day. It is used as a filler and, due to its special white .91 At concentration of 10 mM, it has a pH of 9. Visit https://www. Renal impairment.
Lithium Carbonate was used to treat individuals who are manic depressive. There are many brands and forms of calcium carbonate available. It is a common . A broad range of high-quality products to improve performance, functionality and efficiency. Calcium carbonate is cheapest and therefore often a good first choice.Calciumcarbonat (fachsprachlich), Kalziumkarbonat oder in deutscher Trivialbezeichnung kohlensaurer Kalk, ist eine chemische Verbindung der Elemente Calcium, . It can also be used as cosmetics fillers.
Calcium carbonate
Paper, Plastics, Paints, & Coatings Calcium carbonate is an essential mineral in the paper, plastics, paints, and coatings industries.Calcium carbonate (CaCO 3) is a metal carbonate compound and reacts with hydrochloric acid (HCl) to produce carbon dioxide (CO 2 ), calcium chloride (CaCl 2) and water.calcite, the most common form of natural calcium carbonate (CaCO 3), a widely distributed mineral known for the beautiful development and great variety of its crystals.Products & Solutions. The elements involved are calcium (Ca), carbon (C) and oxygen (O). Calcium Carbonate is limestone.The many faces of calcium carbonate. The most common forms of calcium .Calcium carbonate comes as a tablet, chewable tablet, capsule, and liquid to take by mouth. A person should take it with food, as stomach acid helps the .pH of Calcium Carbonate. Calcium carbonate, CaCO 3, is one of the most common compounds on Earth, making up about 7% of Earth ’ s crust.
calcium carbonate
intlfieldstudie.Calcium carbonate (CaCO 3) makes up nearly 4% of Earth’s crust and is produced mainly by the sedimentation of skeletal remains of marine organisms accumulated over millions of years in the form of, for example, chalk and limestone.Below is a concise summary with things to know about it when it’s used in toothpaste. Navigate to section.
Calcium Carbonate Facts
Note the role calcium carbonate also plays in industrial water .What Is Calcium Carbonate? Calcium carbonate, an alkaline metal carbonate with the formula CaCO 3, is used in various applications from building materials to medical antacids.What is Calcium Carbonate? Calcium carbonate (CaCO3) is an abundant substance found in 4% of the earth’s crust making it one of nature’s more plentiful raw materials.The two main forms of calcium supplements are carbonate and citrate.Structure of Calcium Carbonate.
Products and solutions
Calcium Carbonate (CaCO3)
Calcium carbonate is more commonly available.Calcium carbonate may also be produced by mixing solutions of calcium chloride and sodium carbonate.
- D. Hermann Bollwerk, : Hermann Bollwerk Inhaber Hans Bollwerk
- Känguru. Das Gesundheitsstudio In 85570, Markt Schwaben
- Benedict Cumberbatch : Benedict Cumberbatch
- ¿Por Qué El Agua Se Evapora A Temperatura Ambiente?
- World Journal Of Surgery: Vol 48, No 1
- Quelle Est La Différence Entre Le Conseil D’État Et Le Conseil
- Muskelaufbau Rezepte: Lecker | Muskelaufbau Rezepte: lecker & gesund
- Ristorante Da Luigi, Oldenburg Reisebewertungen
- P2 Abschminkpads, 2 Stück, 3Er Multipack
- Cure Writer’S Block By Janmarie Kelly
- Why Do People Hate Luffy X Nami So Much?
- All Products Offered By The Longhairs
- Aha Garbsen Rückmeldung : Biotonne
- Reina: Baby Name Meaning, Origin, Popularity